Finish
Visual inspection of the finish is completed in order to observe surface conditions, both inside and out, and to check all contact points, welds, junctions, and bend areas. The visual inspection should be completed with an unmagnified eye at the galvanizing facility before the part is shipped.

Several factors can affect the finish of hot-dip galvanized coatings. Some of these factors can be controlled by the galvanizer while others cannot. The specification requirement for finish states the coating must be smooth, uniform, and continuous. There are a number of subjective interpretations of what those words mean, and there are various surface defects that can be visible on a newly galvanized part. Some surface conditions are caused by the design and/or fabrication of the piece and could be eliminated if the best design practices were followed.
There are two primary considerations as to whether a surface condition is acceptable or not according to the specification: effect on corrosion resistance and intended use. The foremost component is whether or not the surface condition has any bearing on the long-term corrosion protection of the part. If the surface condition/defect compromises the life of the coating, it is cause for rejection; however, if it is cosmetic and will not lessen the corrosion resistance, it is acceptable.
The second consideration is the part's intended use. Some surface conditions do not impact the corrosion resistance but make the galvanized part unusable. An example of this would be a handrail with runs and/or dross inclusions, which make the surface bumpy. On another part, this might be acceptable, but because the handrail needs to be smooth for its intended use, this might be cause for rejection.
Once the inspection is complete, any rejected material can be addressed. Material rejected for any reason other than embrittlement can either be repaired or regalvanized and resubmitted for inspection. Repairable areas are restricted by size limitations in the specifications; thus, if the area is too large to repair, stripping and regalvanizing the part is acceptable. Hot-dip galvanizing does not change the mechanical properties of the steel, so this additional strip and regalvanizing does not damage the part.
The following is a list of surface conditions that can be present after galvanizing:
Bare Spots

Bare spots, uncoated areas on the steel surface, can occur because of inadequate surface preparation. Bare spots may be caused by welding slag, sand embedded in castings, excess aluminum in the galvanizing kettle, or lifting devices that prevent the coating from forming in a small area. In order to avoid bare spots, the galvanizer must ensure surfaces are clean and without rust after pretreatment. Small bare spots can be repaired in the galvanizing shop. If the size of the bare spot or the total number of spots causes rejection, the parts may be stripped, regalvanized, and then inspected for compliance with the specifications.
Blasting Damage
Blistered or flaking areas on the surface of the galvanized product due to blasting damage prior to painting of the galvanized steel are caused by incorrect abrasive blasting procedures. These procedures create shattering and delamination of the alloy layers of the zinc coating.

To avoid blasting damage, greatly reduce blast pressured as outlined in ASTM D6386 and D7803 when preparing HDG for painting or powder coating. More information about proper surface preparation of hot-dip galvanized steel for painting or powder coating can be found in the AGA's instructional DVD and guide booklets, Preparing Hot-Dip Galvanized Steel for Paint and Preparing Hot-Dip Galvanized Steel for Powder Coating. Because blasting damage is induced by a post-galvanizing process, the galvanizer is not responsible for the damage.
Chain & Wire Marks

To move steel through the galvanizing process, the steel is lifted by chains and wires attached to overhead cranes. Lifting devices can leave uncoated areas on the finished product that will require repair. Superficial marks left on the galvanized coating from the lifting attachments are not grounds for rejection unless the marks expose bare steel. If bare areas are present, the galvanizer must repair them before the part is acceptable. One potential way to avoid these types of marks is to design permanent or temporary lifting points in the fabrication.
Clogged Holes/Clogged Threads
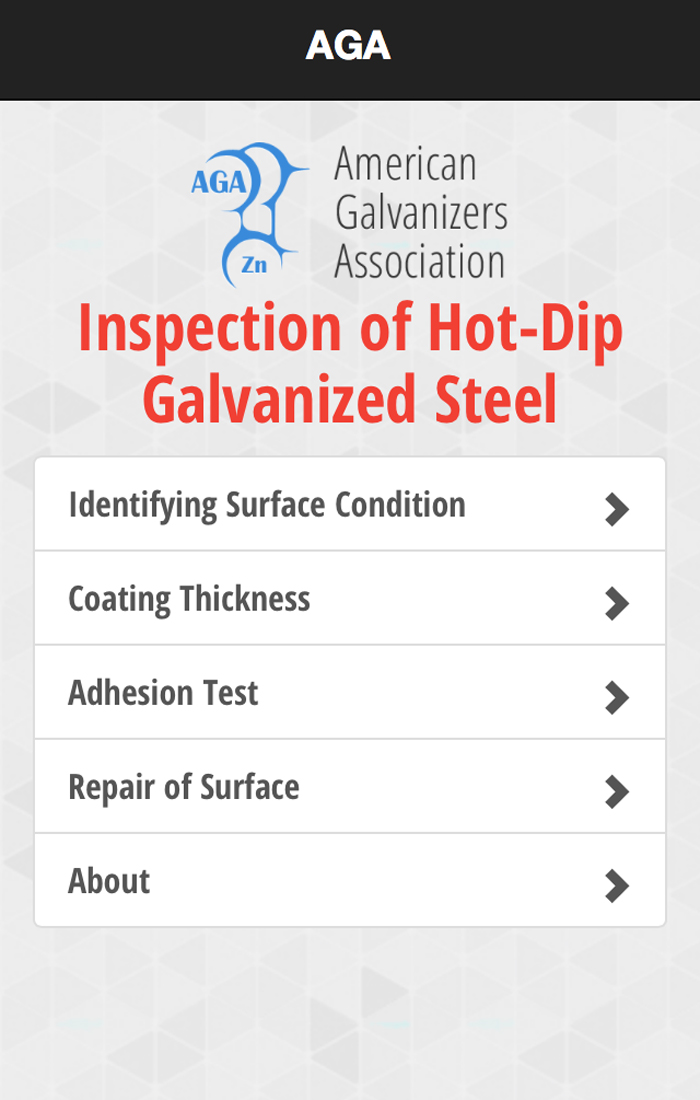
Clogged holes are caused by molten zinc metal not draining adequately and partially or completely filling holes. Molten zinc will not drain easily from holes less than 3/32" (3mm) in diameter due to the viscosity of the metal. A good example is the screen (left). Clogged holes can be minimized by making all holes as large as possible; regardless, clogged holes less than 1/2" (12.7mm) in diameter are not a cause for rejection unless it prevents the part from being used for its intended purpose.
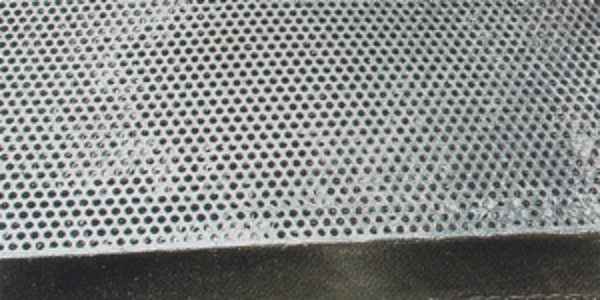
Similarly, clogged threads are caused by poor drainage of a threaded section after the product is withdrawn from the galvanizing kettle. Clogged threads (right) can be cleaned by using post-galvanizing cleaning operations such as a centrifuge or by heating them with a torch to approximately 500 F (260 C) and then brushing them off with a wire brush to remove the excess zinc. Clogged threads must be cleaned and free of excess zinc before the part will meet the specification.
Delamination

Delamination or peeling creates a rough coating on the steel where the zinc has come off. There are a number of causes of zinc peeling. Many large galvanized parts take a long time to cool in the air and continue to form zinc-iron layers after they have been removed from the galvanizing kettle. This continued coating formation leaves behind a void between the top two layers of the galvanized coating. If there are many voids formed, the top layer of zinc can separate from the rest of the coating and peel off the part.
If the remaining coating still meets the minimum specification requirements, then the part is acceptable. If the coating that remains on the steel does not meet the minimum specification requirements, then the part must be rejected and regalvanized. If delamination occurs as a result of fabrication after galvanizing, such as blasting before painting, then the galvanizer is not responsible for the defect.
Distortion

Distortion is the buckling of a thin, flat steel plate or other flat material such as wire mesh. Distortion occurs when steel tries to move to accommodate thermal expansion. Since the steel is welded in place it cannot move. This creates a high-stress level often relieved by distortion of the part. Best practice to avoid distortion dictates fabricating symmetrical parts with similar steel thicknesses and/or temporary bracing. For more on minimizing distortion, see the American Galvanizers Association's (AGA) publication The Design of Products to be Hot-Dip Galvanized after Fabrication or ASTM A384.
Distortion is acceptable unless it prevents the part from fulfilling its intended use. Many distorted thin steel sheets can be bent after galvanizing to bring the part to an acceptable final condition.
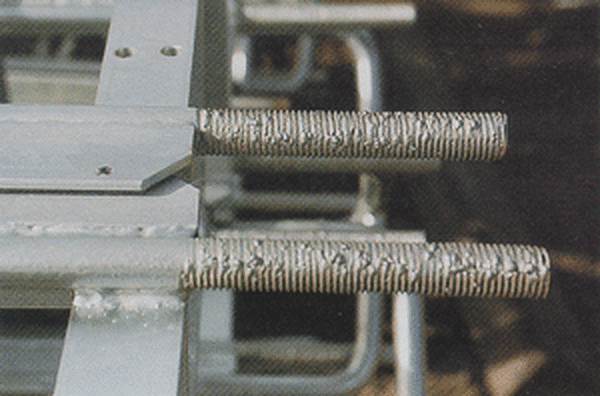
Drainage Spikes
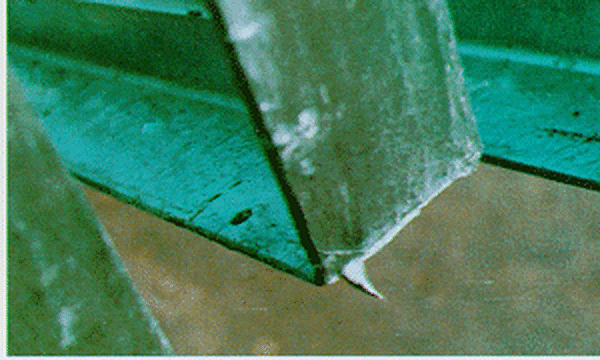
Drainage spikes or drips are teardrops of zinc along the edges of a product. These are caused when steel products are removed from the galvanizing kettle horizontally preventing proper drainage of the zinc from the surface (right). Drainage spikes are typically removed during the inspection stage by a buffing or grinding process. Comprised of excess zinc, drainage spikes and drips will not affect corrosion protection but are potentially dangerous for anyone who handles the parts. Therefore, these defects must be removed before the part can be accepted.
Dross Inclusions

Dross inclusions are a distinct particle of zinc-iron intermetallic alloy that can become entrapped or entrained in the zinc coating. Dross inclusions may be avoided by changing the lifting orientation or redesigning the product to allow for more effective drainage. If the dross particles are small and completely covered by zinc metal, they will not affect the corrosion protection, and thus, are acceptable. If there are gross dross particles (large inclusions) that prevent the full galvanized coating from forming on the steel, then the particles must be removed and the area repaired.
Excess Aluminum in Galvanizing Bath

Galvanizers are required to have a bath of 98% pure zinc, according to the product specifications ASTM A123, A153, and A767, while the remaining 2% is comprised of additives at the galvanizer's discretion. One common additive is aluminum, which helps with the aesthetic of the coating. When excess aluminum is in the galvanizing bath, it creates black marks or bare spots on the surface of the steel. Bare spots due to excess aluminum in the bath may be repaired if only small areas are evident; however, if this condition occurs over the entire part, it must be rejected, stripped, and regalvanized.

Flaking
When heavy coatings (12 mils or more) develop during the galvanizing process, flaking can result. Excessively thick coatings generate high stresses at the interface of the steel and galvanized coating which causes the zinc to become flaky and separate from the steel surface. Flaking can be avoided by minimizing the immersion time in the galvanizing kettle and cooling of the galvanized steel parts as quickly as possible, and/or if possible using a different steel grade. If the area of flaking is small, it can be repaired and then accepted; however, if the flaking area is larger than allowed by the specifications, the part must be rejected and regalvanized.
Flux Inclusions
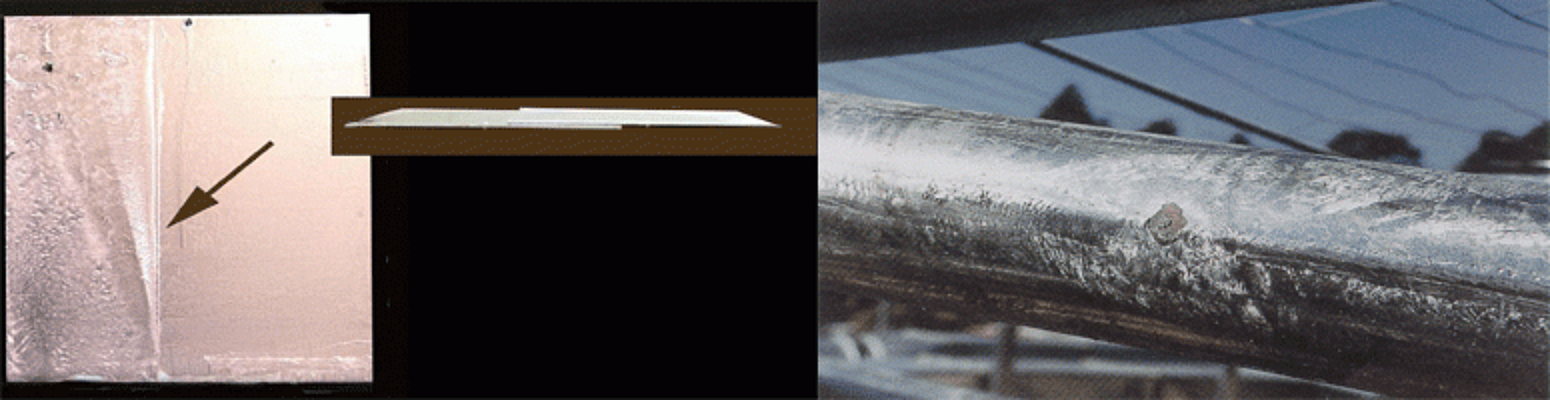
Flux inclusions are created when flux fails to release during the hot-dip galvanizing process, preventing coating formation. Because no coating grows under the inclusion, it must be repaired prior to acceptance. If the area is small enough, it can be cleaned and repaired with touch-up, but if the flux inclusion covers a large area, the part must be rejected. Flux deposits on the interior of a hollow part, such as a pipe or tube, (left) cannot be repaired and must be rejected. Parts rejected for flux deposits may be stripped and regalvanized to provide an acceptable coating.

Oxide Lines
Oxide lines are light colored film lines on the galvanized steel surface created when a product is not removed from the galvanizing kettle at a constant rate. The inconsistent rate of withdrawal may be due to the shape of the product or the drainage conditions.
Oxide lines will fade over time as the entire zinc surface weathers (oxidizes). Strictly an aesthetic condition, oxide lines have no effect on the corrosion performance; and therefore, are not a cause for rejection of hot-dip galvanized parts.
Products in Contact/Touch Marks
Another surface defect can occur if steel parts come in contact with one another or are stuck together during the galvanizing process. This can occur when many small products are hung on the same fixture, creating the chance products may become connected or overlapped during the galvanizing process. The galvanizer is responsible for proper handling of all steel parts in order to avoid defects from products in contact. A similar type of surface defect, touch marks are damaged or uncoated areas on the surface of the product caused by galvanized products resting on one another or by the material handling equipment used during the galvanizing operation. Touch marks are cause for rejection, but may be repaired if their size meets the specification requirement for repairable areas.
Rough Surface Condition
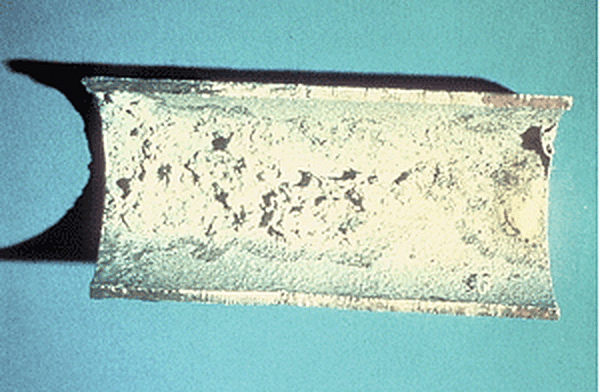
Rough surface condition or appearance is a uniformly textured appearance over the entire product. The cause for rough surface condition could be the steel chemistry or the preparation of the surface by mechanical cleaning, such as blasting before the part reaches the galvanizer. Rough surface condition can actually have a positive effect on corrosion performance because a thicker zinc coating is produced; and therefore, rough coatings are usually not cause for rejection. However, one of the few situations where rough coating is cause for rejection is on handrails, as it impacts the intended use of the product.
Runs
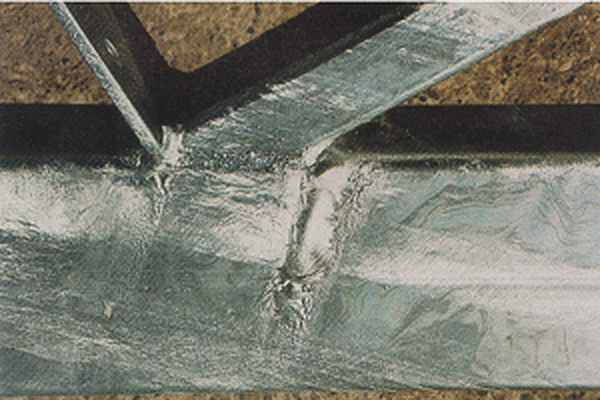
Runs are localized thick areas of zinc on the surface that occur when zinc freezes on the surface of the product during removal from the zinc bath.
Runs are not cause for rejection unless they affect the intended use of the steel part. If runs are unavoidable due to the design of the product, but will interfere with the intended application, they can be buffed.
Rust Bleeding

Rust bleeding appears as a brown or red stain that leaks from unsealed joints after the product has been hot-dip galvanized. It is caused by pre-treatment chemicals that penetrate an unsealed joint. During galvanizing of the product, moisture boils off the trapped treatment chemicals leaving anhydrous crystal residues in the joint. Over time, these crystal residues absorb water from the atmosphere and attack the steel on both surfaces of the joint, creating rust that seeps out of the joint. Rust bleeding can be avoided by seal welding the joint where possible or by leaving a gap greater than 3/32" (2.4mm) wide in order to allow solutions to escape and zinc to penetrate during hot-dip galvanizing. If bleeding occurs, it can be cleaned up by washing the joint after the crystals are hydrolyzed. Bleeding from unsealed joints is not the responsibility of the galvanizer and is not cause for rejection.
Sand Embedded in Castings
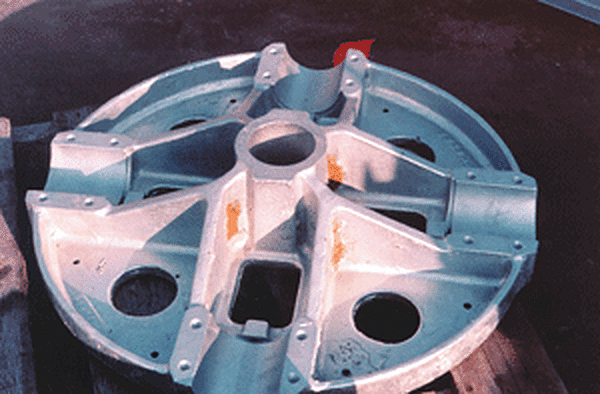
Sand inclusion defects occur when sand becomes embedded in castings and creates rough or bare spots on the surface of the galvanized steel. Sand inclusions are not removed by conventional acid chemical cleaning; therefore, abrasive cleaning should be done before the products are sent to the galvanizer.
Because this defect leaves bare spots, it must be cleaned and repaired, or the part must be rejected, stripped, and regalvanized.
Striations/Fish-Boning
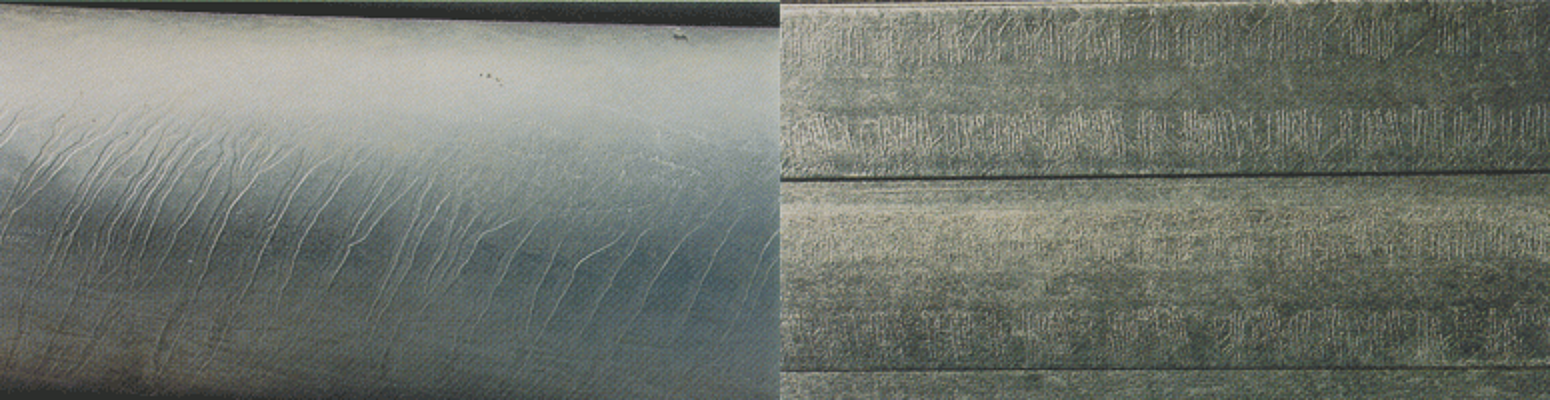
Striations are characterized by raised parallel ridges in the galvanized coating, which can be caused by the chemical composition of the steel. Striations are related to the type of steel that was galvanized, and while the appearance is affected, the performance of the corrosion protection is not striations are acceptable. Fish-boning, similar to striations, is an irregular pattern over the entire surface of the steel part, which is caused by differences in the surface chemistry of a large diameter steel piece and variations in the reaction rate between the steel and molten zinc. These surface conditions do not affect the corrosion performance and are acceptable.
Surface Contaminant

Contaminants on the steel surface not removed by pretreatment will create an ungalvanized area where the contaminant was originally located. Paint, oil, wax, lacquer, or other contaminants chemical cleaning cannot remove cause this; thus, surface contaminants should be mechanically removed prior to the galvanizing process. If they cause bare areas on the final product, they must be repaired. If they meet repairable size limits in the specification, they may be repaired; however, if the repair is too large, the part must be rejected and regalvanized.
Weeping Weld
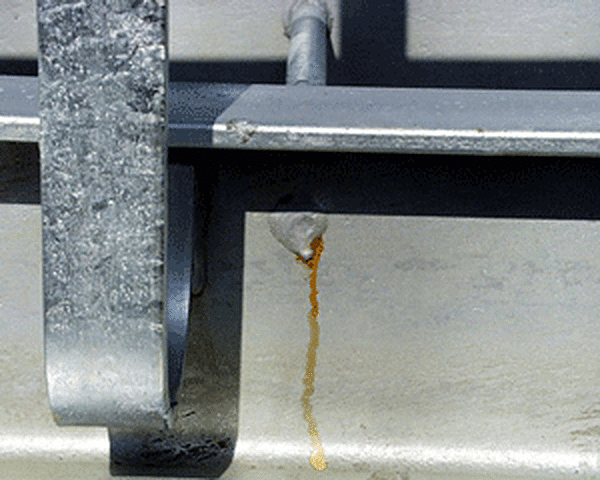
Weeping welds stain the zinc surface at welded connections on the steel. Caused by entrapped cleaning solutions that penetrate the space between the two pieces, weeping welds can be avoided by providing a 3/32" (2.4mm) or larger gap between the two pieces when welding them. This will allow the zinc to penetrate the gap.
A weld made with gaps instead of continuous weld bead, actually makes a stronger joint when the process is complete. Weeping welds are not the responsibility of the galvanizer and are not cause for rejection.
Welding Blowouts

Welding blowout is a bare spot around a weld or overlapping surface hole caused by pre-treatment liquids penetrating the sealed and overlapped areas that boil out during immersion in the liquid zinc. Blowouts cause localized surface contamination and prevent the galvanized coating from forming. In order to avoid welding blowouts, check weld areas for complete welds to ensure there is no fluid penetration. In addition, products can be preheated prior to immersion into the galvanizing kettle in order to dry out overlap areas as much as possible. Bare areas caused by welding blowouts must be repaired before the part is acceptable.
Welding Spatter
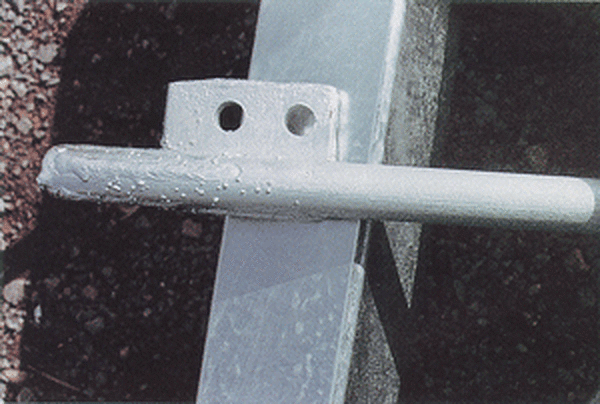
Welding spatter appears as lumps in the galvanized coating adjacent to weld areas due to spatter left on the surface of the part from fabrication. To avoid welding spatter, welding residues should be removed prior to hot-dip galvanizing. Welding spatter appears to be covered by the zinc coating, but the coating does not adhere well and can be easily removed leaving an uncoated area or bare spot. If this defect occurs, the area must be cleaned and properly repaired, which may require regalvanizing.
Wet Storage Stain

Wet storage stain is a white, powdery surface deposit on freshly galvanized surfaces. Wet storage stain is caused by newly galvanized surfaces being covered by moisture, such as rain, dew, or condensation and having no airflow over the surface. Water reacts with the zinc metal on the surface to form zinc oxide and zinc hydroxide. Wet storage stain is most often found on stacked and bundled items, such as galvanized sheets, plates, angles, and bars. It can have the appearance of light, medium, or heavy white powder on the galvanized steel product.

One method to avoid wet storage stain is to passivate the product after galvanizing with a chromate quench solution. Also, avoid stacking products in poorly ventilated, damp conditions. Light or medium wet storage stain will weather over time in service and is acceptable. In most cases, wet storage stain does not indicate degradation of the zinc coating, nor does it imply any likely reduction in the expected life of the product; however, heavy wet storage stain should be removed mechanically or with chemical treatments before the galvanized part is put into service.
For more information on removing wet storage stain, please see the AGA's Galvanizing Note, Cleaning Wet Storage Stain from Galvanized Surfaces.
Zinc Skimmings

Zinc skimming deposits are usually caused when there is no access to remove the zinc skimmings during the withdrawal of the steel from the galvanizing kettle. Zinc skimmings on the molten zinc surface are then trapped on the zinc coating. Zinc skimming deposits are not grounds for rejection as long as the zinc coating underneath is not harmed during their removal and it meets the necessary specifications.
Zinc Splatter

Zinc splatter is defined as splashes and flakes of zinc that loosely adhere to the galvanized coating surface. Zinc splatter is created when moisture on the surface of the galvanizing kettle causes liquid zinc to "pop" and splash droplets onto the product. These splashes create flakes of zinc loosely adherent to the galvanized surface. Zinc splatter will not affect the corrosion performance of the zinc coating and thus is not cause for rejection. The splatter does not need to be cleaned off the zinc coating surface, but can be if a consistent, smooth coating is required.