Galvanic Corrosion
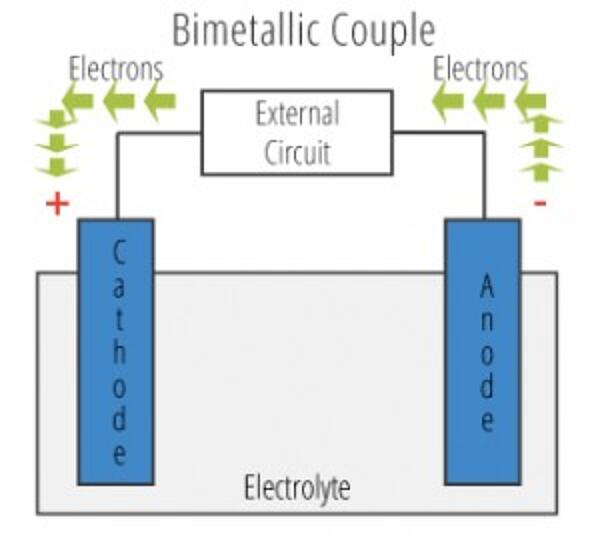
There are two primary types of galvanic cells that cause corrosion: the bi-metallic couple and the concentration cell. A bi-metallic couple is like a battery, consisting of two dissimilar metals immersed in an electrolyte solution. An electric current (flow of electrons) is generated when the two electrodes are connected by an external, conductive path.
A concentration cell consists of an anode and cathode of the same metal or alloy and a return current path. The electromotive force is provided by a difference in concentration of the surfaces through the external path.
There are four elements necessary for corrosion to occur in a galvanic cell:
- Anode - The electrode where galvanic reaction(s) generate electrons - negative ions are discharged and positive ions are formed. Corrosion occurs at the anode.
- Cathode - The electrode that receives electrons - positive ions are discharged, negative ions are formed. The cathode is protected from corrosion.
- Electrolyte - The conductor through which current is carried.. Electrolytes include aqueous solutions or other liquids.
- Return Current Path - The metallic pathway connecting the anode to the cathode. It is often the underlying metal substrate.

All four elements (anode, cathode, electryolyte, and return current path) are necessary for corrosion to occur. Removing any one of these elements will stop the current flow and galvanic corrosion will not occur. Substituting a different metal for the anode or cathode may cause the direction of the current to reverse, resulting in a switch to the electrode experiencing corrosion.
The Galvanic Series of Metals (right) lists metals and alloys in decreasing order of electrical activity. Metals nearer the top of the table are less noble metals and have a greater tendency to lose electrons than the more noble metals found lower on the list.
