Galvanized Steel with Aluminum Parts
What happens when I put galvanized steel together with aluminum parts or put aluminum sheets on galvanized steel studs?
The question is frequently asked when two dissimilar metals are placed in contact with each other. The chart below shows the electrode potentials of metals in seawater. If two metals with different potentials are placed in contact and there is a conductive medium, such as sea water or condensation, there can be a reaction, commonly known as a galvanic cell.
The higher the electrical potential difference the greater the possibility of a reaction. In the case of zinc and aluminum, there is only a slight chance of a reaction because of the relatively small change in potential between the two metals and the formation of an insulating film on the surface of the aluminum.
One of the key factors in the reaction between dissimilar metals is the contact surface area. A severe reaction can occur when a large cathode (higher or more positive potential) is in contact with a small anode (lower or negative potential). In this situation, corrosion rates can increase dramatically.
Another key factor in the determination of corrosion of two dissimilar metals in the presence of conductive substances. In many cases, condensed water does not provide enough conductance to start the corrosion process. Whenever possible, the best solution to this type of corrosion is to prove an insulation barrier between the two dissimilar metals.
For more info on hot-dip galvanized steel in contact with dissimilar metals, refer to AGA guide on dissimilar metals.
Electrode Potentials of Metal in Sea Water
| Material | Potentail (volts) |
|---|---|
| Magnesium | -1.55 |
| Zinc | -1.10 |
| Aluminium | -0.86 |
| Cadmium | -0.77 |
| Cast Iron | -0.68 |
| Carbon Steel | -0.68 |
| Stainless Steel 18% CR 8% Ni (active) | -0.61 |
| Lead | -0.57 |
| Solder (50Pb/50Sn) | -0.52 |
| Tin | -0.49 |
| Copper | -0.43 |
| Aluminium Bronze | -0.41 |
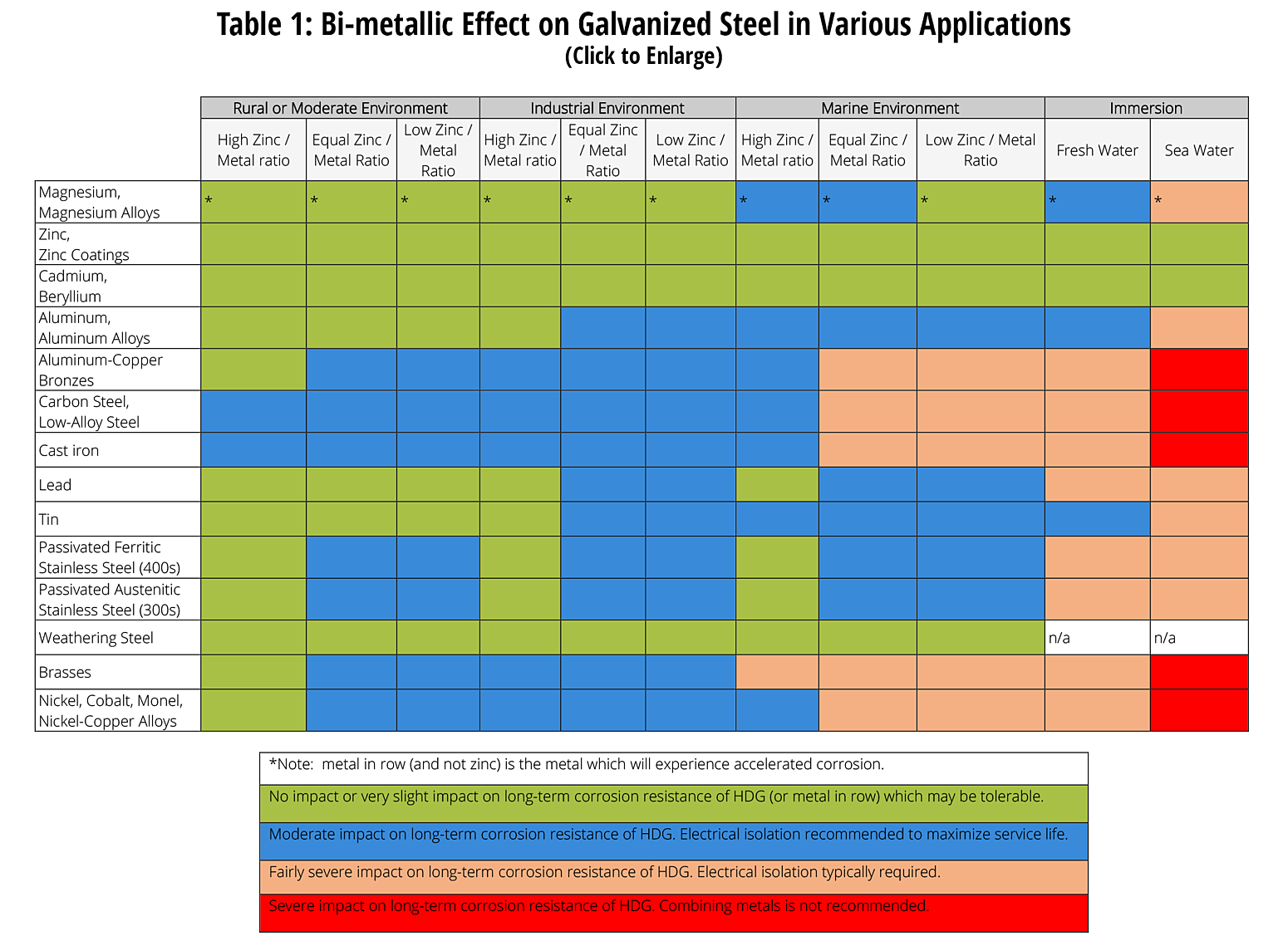
For more detailed information about galvanic corrosion and hot-dip galvanizing in contact with dissimilar metals, see Dissimilar Metals in Contact.
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.

