Steel Corrosion Process
The corrosion process that takes place on a piece of uncoated steel is very complex. Factors such as variations in the composition/structure of the steel, presence of impurities due to the higher instance of recycled steel, uneven internal stress, and/or exposure to non-uniform environment all affect the corrosion process.
It is very easy for microscopic areas of the exposed steel to become relatively anodic or cathodic to one another. A large number of such areas can develop in a small section of the exposed steel. Further, it is highly possible several different types of galvanic corrosion cells are present in the same small area of an actively corroding piece of steel.
As the corrosion process progresses, corrosion products tend to build up in certain areas of the metal. These corrosion products have different elemental compositions than the original state. The new compositions exposed on the surface lead to changes in the anodic and cathodic areas. As the change in anodic and cathodic areas occur, previously uncorroded areas of the metal can be attacked and corrode. This will accelerate the overall corrosion of the steel surface.
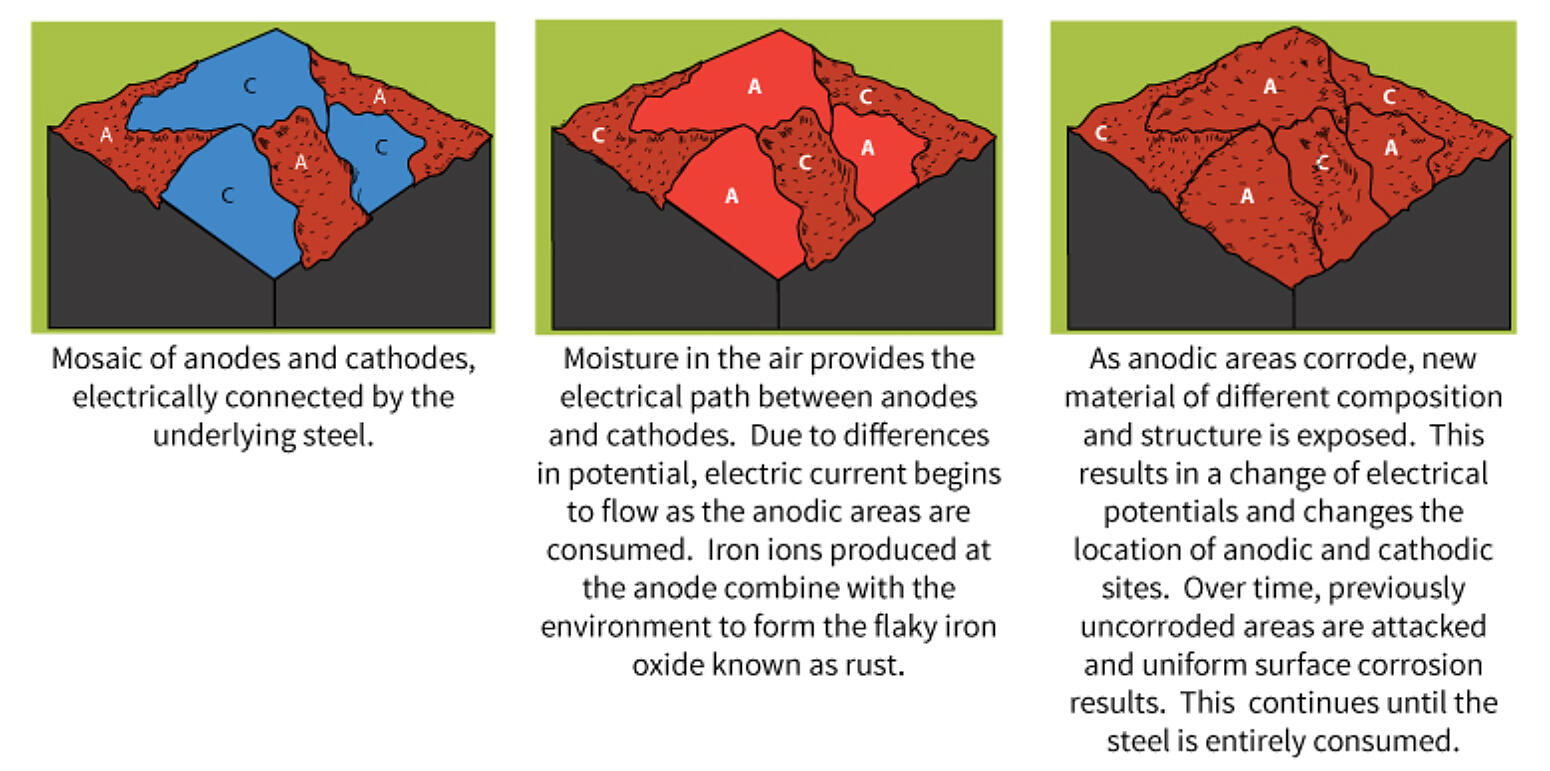
The corrosion products of steel are oxide particles and have a distinctive brown/red (rust) color. Just a small amount of these particles can cause an uncoated steel surface to appear corroded. Steel corrodes naturally when exposed to the atmosphere, but the corrosion process accelerates when electrochemical corrosion cells are active on the surface.
