Dissimilar Metals in Concrete
*Updated 2026
Galvanic corrosion is a potential concern when hot-dip galvanized steel is combined with other metals and embedded in concrete, but there are several methods to prevent it. If galvanized steel and uncoated steel are to be connected or in close proximity in concrete, accelerated corrosion of the zinc coating may occur after depassivation of the galvanized rebar in concrete. Though depassivation of zinc does not often occur for many decades, efforts to prevent galvanic corrosion later on are important to consider for applications in marine environments or for bridge decks exposed to heavy road salting.
Galvanic corrosion requires four simultaneous conditions: an anode, a cathode, an electrolyte, and an electrical return path. When these conditions exist, a corrosion cell is formed (Figure 1), enabling galvanic corrosion to occur. Although it may take decades for moisture to permeate the concrete and contact the reinforcement embedded within it, if dissimilar metals are contacted a corrosion cell can form.
For more information on hot-dip galvanized steel in contact with dissimilar metals, refer to AGA guide on Dissimilar Metal Corrosion with Zinc.
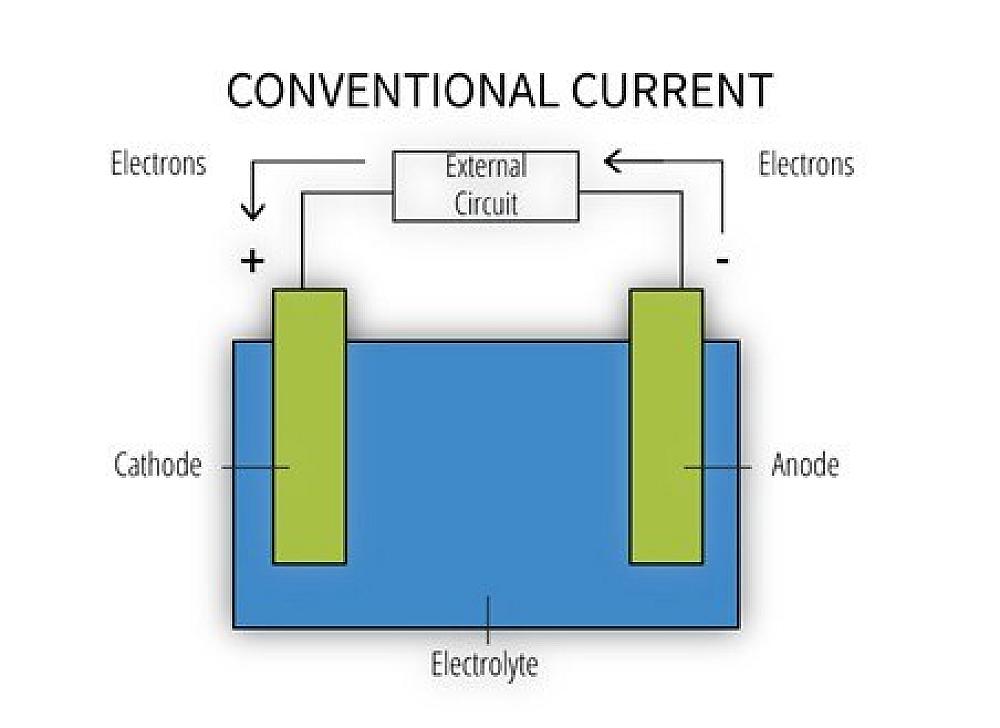
Concrete is porous in nature and therefore allows small amounts of water to seep into its matrix with time. This means materials selection is an important consideration for reinforcing steel, couplers, tying wires, supports, and any other steel within the concrete. If two or more of the metals are dissimilar, a corrosion cell will form once the water (electrolyte) contacts the anode and cathode (Figure 2) completing the circuit.
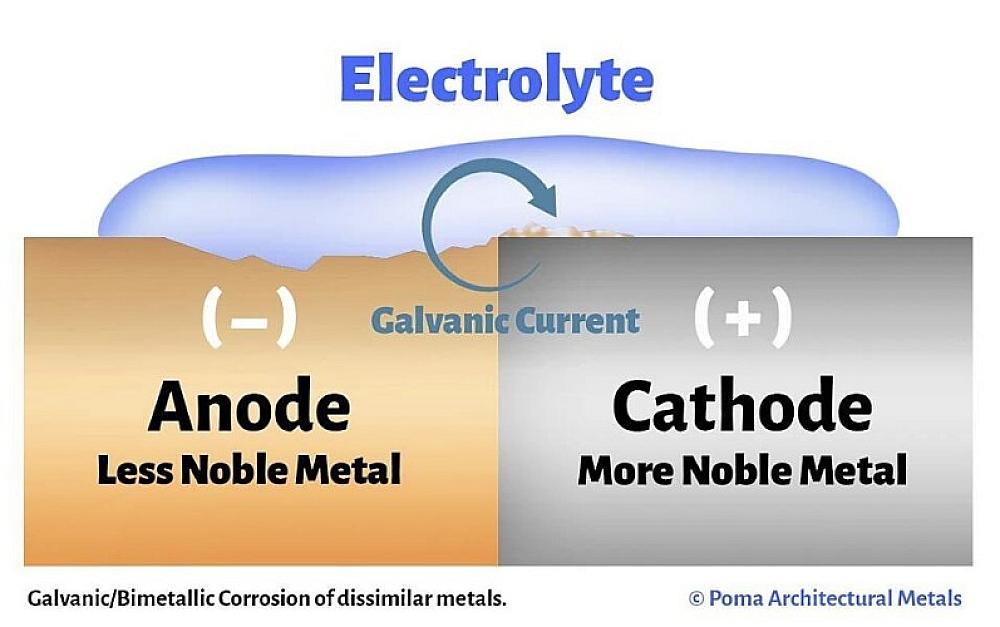
The easiest and most effective way to prevent galvanic corrosion is to stop the corrosion cell from forming. There are three ways to accomplish this for reinforced concrete.
Ideally, use one material—such as hot-dip galvanized (HDG) steel—for all reinforcement. Eliminating the anode–cathode relationship removes the possibility of galvanic corrosion.
If uniform materials are not an option, and dissimilar metals must be used (e.g. repairs and retrofits), isolating the dissimilar metals in contact will also prevent corrosion by disrupting the corrosion cell. This can be done with electrical tape, plastic sleeves, insulated ties, and/or foam spray or rigid foam supports.
Another method to mitigate galvanic corrosion is to place mixed connections well below the surface of the concrete to remove or limit exposure to an electrolyte. However, this approach may not always be feasible, as increasing cover depth can add weight and cost to a project.
If you would like to learn more about embedding hot-dip galvanized rebar in concrete, or have more questions about galvanic corrosion, please contact the AGA technical department at 720-361-4485 or [email protected].
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.


