Stainless Steel in Contact with Galvanized Steel
Stainless steel and galvanized materials often are found together in industry with applications such as galvanized fasteners, stainless steel pressure vessels and roof and siding panels. Do I need to worry about these two metals corroding each other? What other concerns should I have pertaining to hot dip galvanized steel and stainless steel in contact?
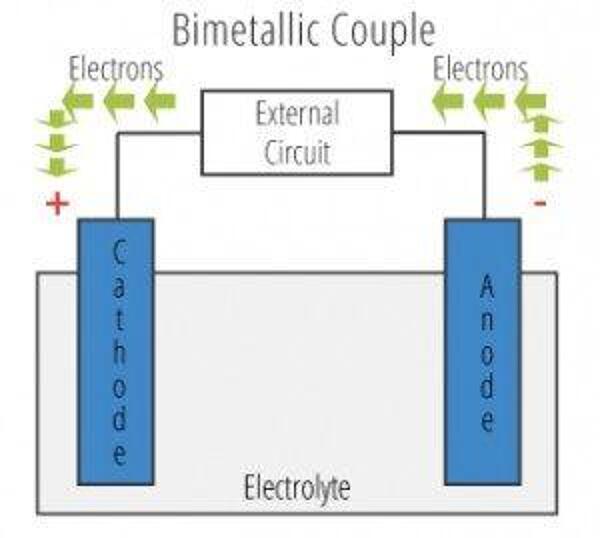
Stainless steel and galvanized materials often are found together in the industry with applications such as galvanized fasteners, stainless steel pressure vessels and roof and siding panels. The presence of two dissimilar metals in an assembly is not always a sign of trouble but it could be a problem. When two metals are in direct contact, there is the potential for the formation of a bimetallic couple. There are four elements necessary for the contact metals to experience corrosion;
- One of the metals must act as the anode and generate electrons that can create an electrical current flow.
- The other metal must act as a cathode and collect these flowing electrons. This metal is the protected partner of the corrosion cell.
- There must be an electrolyte material covering these two metals at the area where they are touching to complete the electrical current path. This electrolyte material must be able to conduct ions from one metal to the other; and
- There must be a return current path which in almost all cases is a direct contact between the two metals. The following diagram shows all of the parts of the bimetallic couple.
As in all design and fabrication situations, the problem is not as simple as just looking it up on a chart. The zinc has been applied to the steel to provide corrosion protection for the underlying base steel. If zinc is in contact on the surface with a more cathodic metal and the zinc becomes part of a bimetallic couple and corrodes, then the zinc is not performing its designed function of protecting the base steel. The formation of a bimetallic couple needs four elements in order to form. The existence of two dissimilar metals in direct contact can be no problem whatsoever if there is no electrolytic material present.

In most atmospheric applications the only potential electrolytic material that can be present is rainwater or dew. Both of these forms of water are poor electrolytic materials since they do not contain many salts and ions which would make them conductive. On the other hand, marine environments and areas where the melting snow includes road salts can be very good electrolyte materials. Bimetallic couples are more easily formed in immersion situations where the assembly will be underwater when it is in service. Salt water is especially tough on two dissimilar metals in contact. The best guide as to how various metals will react in contact with zinc under different environments is the following table.
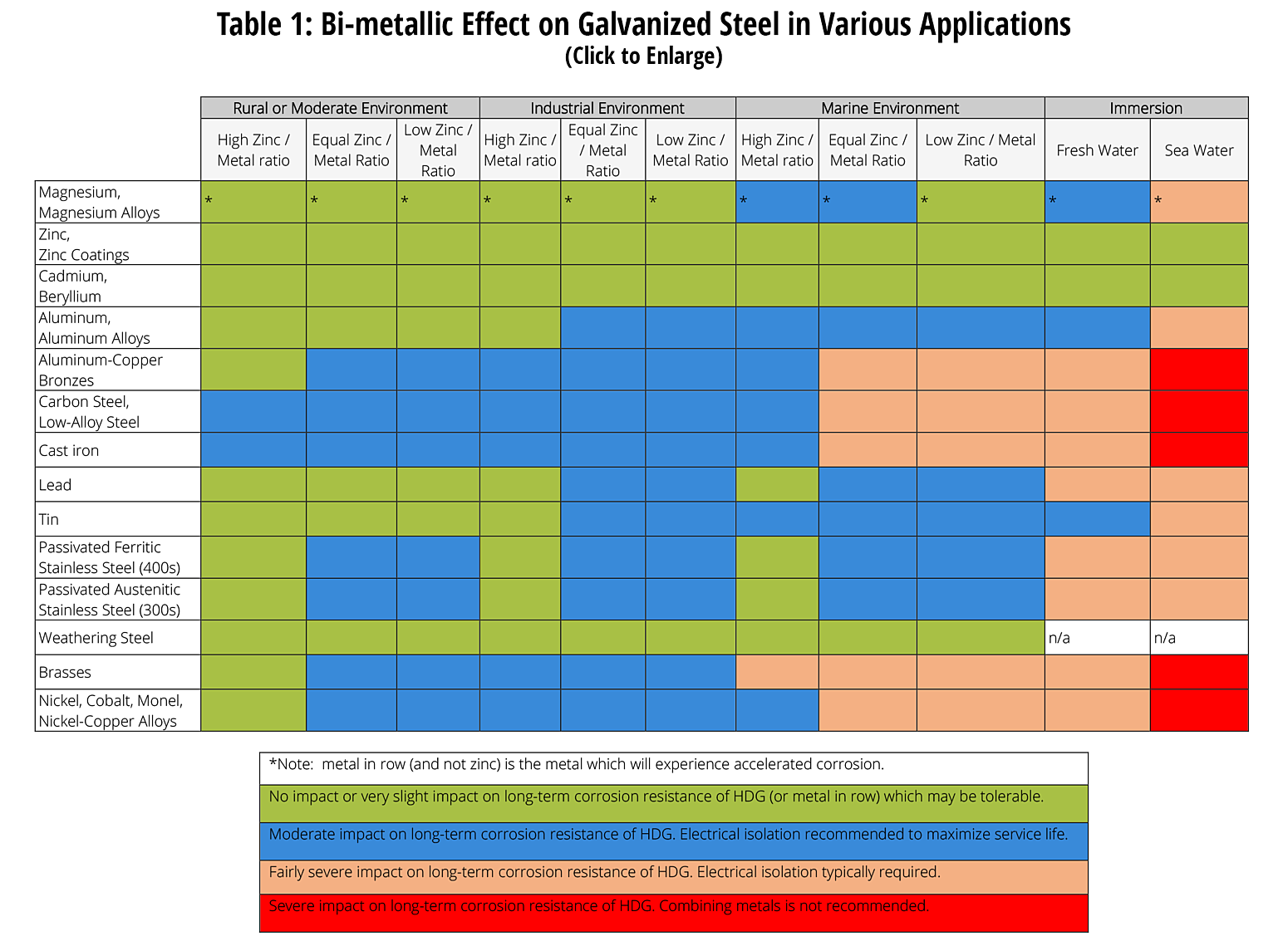
The performance of zinc in contact with most of the common building metals is rated for most environments. This figure is easy to understand and provides a good reference sheet to fax to those who are concerned with the potential of forming a bimetallic cell.
The final answer to those who want to assemble systems with dissimilar metals that will be immersed in service is to electrically isolate the two pieces by inserting an insulating material between them. The breaking of the contact between the two metals will effectively stop any possibility of forming a bimetallic cell. Most plastics are good insulating materials. For saltwater immersion, the most common insulator is a piece of rubber.
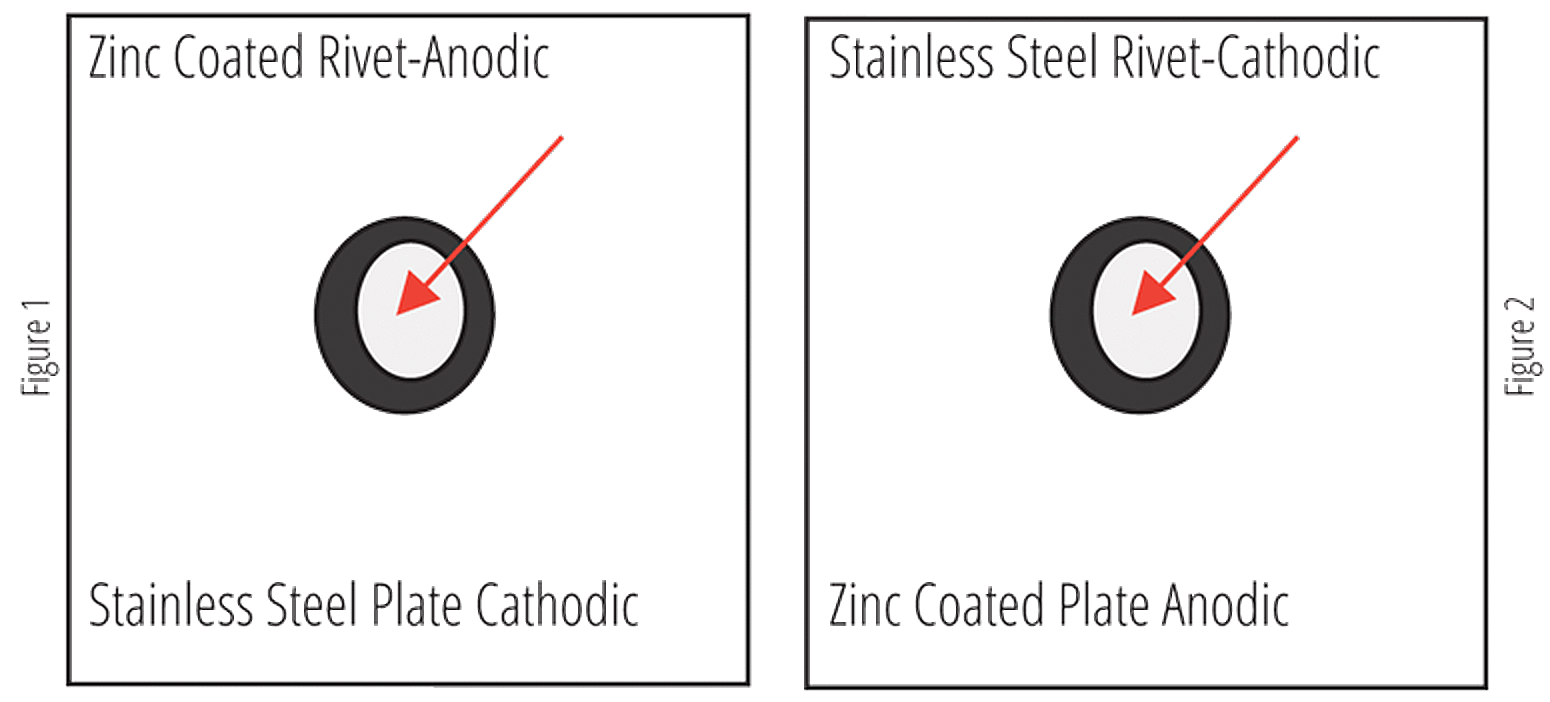
One Exception
The rate of penetration of corrosion increases as the ratio of the cathode to anode surface area increases; as it decreases, the rate of penetration decreases. This situation is portrayed using a riveted fastener as shown in Figures 1 and 2. When using a stainless steel plate with a zinc rivet (Fig. 1), the ratio of the cathode surface area to the anode surface area is large, and the rivet will fail rapidly because of accelerated corrosion. When combining a zinc plate with a stainless steel rivet (Fig. 2), the area ratio between the cathode and anode is reversed, and although more surface area is affected, the depth of penetration is small; the fastener should not fail because of corrosion. The size correlation to the corrosion rate is also shown in Table 1.
| Surface area of metal in row relative to Surface area in column | Zinc | Stainless Steel | |
|---|---|---|---|
| Zinc | Small Large | -- -- | S G |
| Stainless Steel | Small Large | G G | -- -- |
For more detailed information about galvanic corrosion and hot-dip galvanizing in contact with dissimilar metals, see Dissimilar Metals in Contact.
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.
