Evaluating Steel Chemistry Prior to Galvanizing
What kind of coating can I expect when galvanizing an unfamiliar steel grade?
Most iron-containing materials are suitable for galvanizing, but how do you know what to expect when galvanizing an unfamiliar steel grade? Evaluation of the steel chemistry is critical in understanding if and how the material will galvanize. When this evaluation is performed by the galvanizer and the engineer/specifier together, steel chemistry can either be optimized in advance when ordering from the mill or adjustments can be made in the galvanizing process with the intent of meeting everyones expectations regarding final coating appearance and thickness.
Evaluating Steel Chemistry
To evaluate the chemistry for an unfamiliar steel grade, the elemental composition of the steel in weight percentage should be estimated from the mill test reports for the heat. Where mill reports (or accurate mill reports) cannot be obtained, it is possible to locate a range for each element in the steel by looking up Chemical Requirements tables within the steel grade specification or by acquiring ranges from the steel manufacturers website/brochure. The elements listed in ASTM A385 should be compared according to the recommendations in order to achieve a coating which is of typical appearance and thickness.
TABLE 1: RECOMMENDED ELEMENTAL COMPOSITIONS FOR HOT-DIP GALVANIZING (REF. ASTM A385 SECTION 3.2)
| Element | Recommended % for HDG | Notes |
|---|---|---|
| Si | < 0.04 % or 0.15% - 0.22% | Sandelin steels and steels high in Si content may produce thick, matte, and/or rough coatings |
| P | < 0.04% | P > 0.04% produce rough, thick coatings susceptible to delamination |
| Si Equivalent | < 0.04 % or 0.15% - 0.22% | Si Equivalent 0.04% - 0.15% or > 0.22% may produce thick, matte, and/or rough coatings |
| C | < 0.25% | Check ultimate tensile strength for steels >1% C |
| Mn | < 1.3% | High Mn may produce brownish colored and brittle coatings |
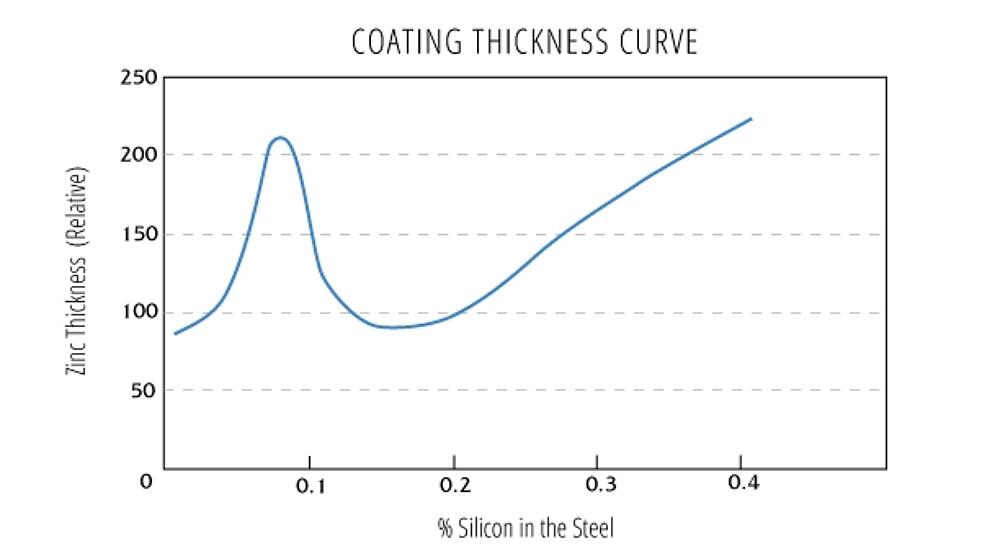
The Silicon Equivalent
Steels with silicon and phosphorus levels which are beyond the recommended levels are considered reactive steels. Reactive steels are galvanized regularly, and the silicon equivalent is used to evaluate the combined effect silicon and phosphorus have on the metallurgical reaction during hot-dip galvanizing. After the Silicon equivalent has been calculated, look up the value on the Sandelin Curve to predict the reactivity of the steel.
Silicon Equivalent = Si content + 2.5*(P content)
- Silicon equivalent <0.04% or 0.15% - 0.22% - coatings are of typical thickness and appearance.
- Silicon equivalent 0.04% - 0.15% (Sandelin steels) - vary in appearance and develop thicker coatings. If a nickel alloyed zinc bath is used, the Sandelin effect is mitigated, resulting in a higher likelihood of bright coatings of typical coating thickness.
- Silicon equivalent > 0.22% - matte gray and rough coatings of a thickness greater than the minimum requirements. See Sandelin curve to determine the level of reactivity and anticipated coating thickness.
Stainless Steels
Stainless steels of the 300 series can be galvanized because they contain nickel, which is necessary to initiate the reaction between the steel and the zinc. Alternatively, stainless steels of the 400 series do not contain nickel and cannot be galvanized successfully.
Weathering Steels
Weathering steels (ASTM A588, A709 Weathering, COR-TEN) typically develop thicker galvanized coatings due to higher levels of silicon (up to 0.40% allowed, and 0.27% to 0.35% common). Additionally, due to the initial surface roughness, coating weights are comparable for both pickled and blast-cleaned surfaces. Expect a matte gray coating with little or no spangle.
Low Silicon / Aluminum-killed Steels
Steels with very low levels of silicon (less than 0.02%) or aluminum-killed steels regularly present a challenge in developing a coating that meets the thickness requirements of ASTM A123 and A153.
Copper-Containing Steels
Some steel chemistries with small amounts of copper such as weathering steels can be successfully hot-dip galvanized. Pure copper cannot be galvanized. Coatings which are thicker and darker in appearance are typical.
High-Speed Machining Steels
Steels containing high amounts of sulfur (S >0.18%) are unsuitable for hot-dip galvanizing and will erode.
Other Considerations
There are additional considerations when evaluating steel chemistry, as predicting steel reactivity is not an exact science. Element levels can vary +/- 0.02% and the values listed on the mill test report are only one sample taken from the heat. The element levels on the individual pieces, and even on the same piece of steel, can vary to some degree. However, using mill reports which state the estimated elemental composition typically leads to the most accurate evaluation. Additionally, be aware that some foreign steel producers are known to have inaccurate steel chemistry analyses on the mill reports. When in doubt, galvanize a test sample for confirmation.
Once the steel chemistry has been evaluated, be sure to also evaluate ultimate tensile strength, initial surface condition, the use of old or recycled steel, surface roughening, steel thickness, and the presence of any thermally cut edges to determine whether any of these conditions may have additional effects on the final coating thickness and appearance.
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.

