Thinner Galvanized Coatings
How do I get thinner galvanized coatings on my steel?
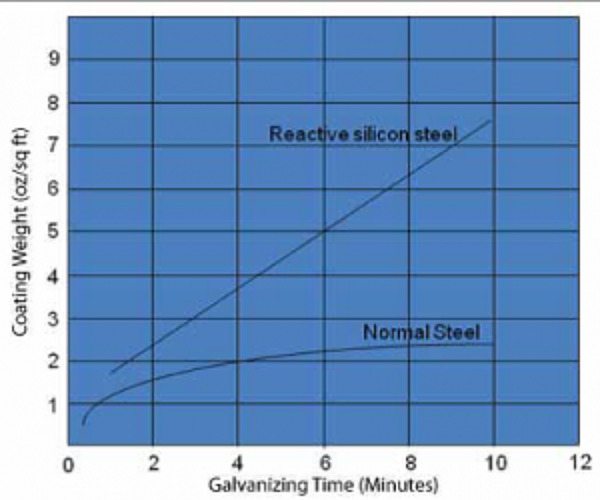
This question has come up with more frequency lately from galvanizers, possibly because reducing zinc consumption has become more important as belts have been tightened during the recession the last few years. The best place to start the conversation about achieving thinner coatings is by talking about steel chemistry since that has the biggest influence on coating thickness. For most types of non-reactive steel, which is steel that meets the chemistry recommendations listed in ASTM A385, you will not have to worry much about excessively thick galvanized coatings, because, after a few minutes in the galvanizing kettle, coating growth levels out (see Figure 1).
That is not the case with reactive steel, which is steel that falls in the Sandelin Range or has a silicon equivalent (see below) greater than about 0.25%. For these types of steels, coating growth continues as long as the steel is left in the kettle (see Figure 1).
Silicon Equivalent = Si% in steel + 2.5(P% in steel)
So, the first step to achieving thinner galvanized coatings is to identify when you have reactive steel. Sometimes that is as straightforward as looking at the steel certification, but other times it has to be determined by galvanizing the steel because steel certifications do not always accurately reflect the steel your customer gives you.
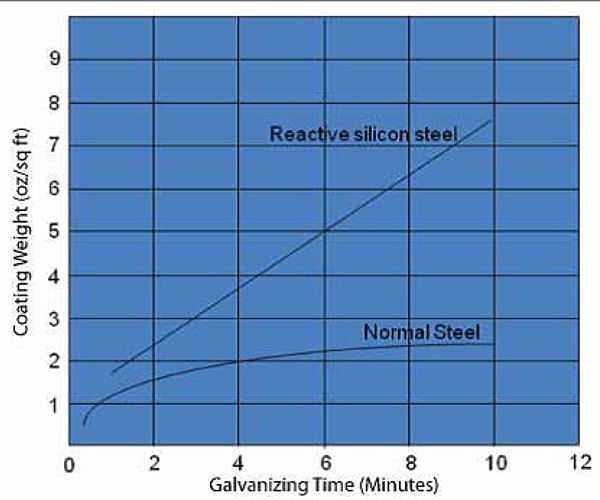
If you know your customers steel is reactive, sweep blasting it is the surest way galvanizers have to reduce excessively thick coatings. Blasting the steel roughens the surface, which causes the zinc crystals to grow into one another rather than growing vertically like on steel that has not been blasted. The thickness of the galvanized coating is reduced when the zinc crystals grow into one another. When you know you have reactive steel, another thing you can do is leave the steel in the kettle for a shorter period of time. This is not always possible, for instance, when galvanizing very thick steel which must be left in the kettle longer to achieve the necessary temperature for the metallurgical reaction to happen. Notice in Figures 1 and 2, coating growth is proportional to the time the steel is in the kettle. Another way to limit coating thickness is by reducing the kettle temperature. Higher temperatures help the galvanizing reaction to happen faster. The corollary is lower galvanizing temperatures tends to cause slower reactions, which can reduce coating thickness.
A final option for reducing coating thickness is with bath element additions. Nickel reduces the growth of the intermetallic layers in steels with chemistry that falls into the Sandelin Range. Lead, an element found in Prime Western zinc, reduces the surface tension of zinc, which helps the zinc to drain off the steel more easily as the part is being removed from the kettle. Bismuth accomplishes this same task and is sometimes used in kettles with High Grade or Special High-Grade zinc that have much less lead than Prime Western zinc. (Please see the AGA Galvanizing Note on kettle chemistry for more information about bath element additions.) So where is the best place to start to reduce galvanized coating thickness? In a perfect world, you could explain to your customers that they need to supply you with steel with chemistry similar to that recommended in ASTM A385, and they would. But that is rarely the case since there is a lot of reactive steel on the market today.
So, addressing the chemistry of your bath to adjust for reactive steel is a good place to start. Next, you could communicate to your customers how blasting the steel prior to galvanizing is sometimes necessary to achieve a galvanized coating that is not excessively thick. Lastly, decreasing time in kettle and kettle temperature are other options for reducing coating thickness. Although none of these methods guarantee a thinner coating, they are your best options for accomplishing the goal.
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.

