Galvanized Steel Performance - pH Curve
How does Galvanized steel stand up to the corrosion challenge in special conditions?
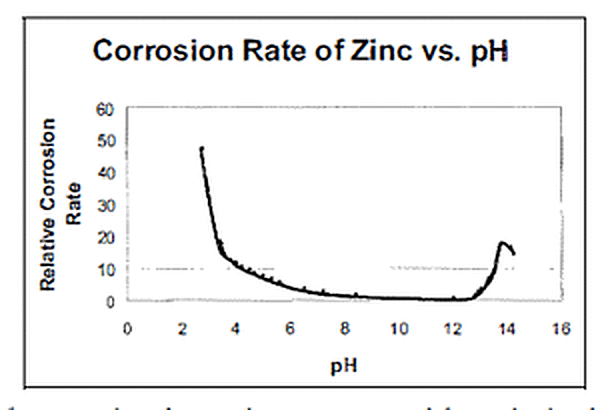
The pH of a solution identifies the concentration of hydrogen ions in the fluid. Acids, which have a low ph have a high concentration of hydrogen ions. Based or alkaline solutions have a high pH, which relates to low concentrations of hydrogen ions. The pH of a solution will dictate the solubility of other constituents in the solution. Some solids are more soluble in acidic solutions while other solids are more soluble in basic solutions. In certain cases, as with zinc oxide, species may exhibit both acidic and basic solubility. These species are called amphoteric.
After a newly galvanized part is removed from the kettle, the foundation of a protective layer on the zinc metal begins. The outer layer of free zinc reacts with oxygen in the atmosphere to produce zinc oxide. Since zinc oxides are amphoteric, this layer exhibits both acidic and basic solubility. Thus, the stable region in which zinc exhibits the least corrosive behavior is in neutral solutions.
Galvanized steel performs best in solutions with a pH in the range of 5.5 to 12. pHs between 3 and 5.5 (acidic) or 12 and 13.5 (basic) are corrosive to galvanized steel, but the galvanized coating will still give corrosion protection to bare steel, although the protection will only last for a few years. If a longer service life is desired, then a duplex system using an acid or base resistant paint or epoxy over the galvanized coating is recommended. In the figure, youll see the relative corrosion rate of galvanized steel related to the pH of its environment. For example, say you had a 4 mil thick coating; youd get about five times as much service life from this coating at pH 3.5 as you would at an approximate pH 2.5. Chemical environments with pHs below 3 or above 13.5 are not recommended for galvanized steel due to the rapid corrosion of the galvanized coating. Other corrosion protection systems that can be used in these chemical environments where galvanized steel is not recommended include stainless steel or polymers. In addition to pH, corrosion of galvanized coatings is also affected by the concentration of the acid or base in the solution, agitation, aeration, temperature, polarization and if inhibitors are used.
Examples
Galvanized steel containers are widely used for storing and transporting chemical solutions. Many organic solutions are relatively neutral; thus galvanized steel provides corrosion protection without affecting the integrity. A list of over 600 chemicals that have been successfully stored in galvanized steel containers is available in Corrosion Resistance of Zinc by Frank Porter "Great! The technical data looks good, but how about a few examples of some applications where I might not think galvanized steel performs well?"
One application where galvanized steel has performed particularly well is in Sterling Chemicals' petrochemical plant in Texas City, Texas. the galvanized pipe racks in the plant have been in service for 30 years and some of them have retained a coating thickness that exceeds ASTM thickness requirements for newly galvanized steel The galvanized steel has exhibited excellent durability despite the harsh industrial and coastal environment.
Another application where galvanized steel has been successful in fertilizer plants. Fertilizer plants produce anhydrous ammonia and phosphate-containing compounds. In the absence of excessive heat and moisture, galvanized steel performs very well in the presence of these chemicals. One such ammonia plant, Joffre Nitrogen Operations in Joffre, Alberta, has erected a majority of its steel structures using hot-dip galvanized. Construction was completed in 1987 and the current condition of the galvanized structures are excellent.
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.

