Brown Staining on Galvanized Coatings
I recently received a hot-dip galvanized product with brown stains on the surface. How are these stains created and what can be done to minimize their formation? Is this type of surface defect acceptable according to the ASTMA123 specification?

A hot-dip galvanized product may develop a surface defect known as brown staining, which is created when the iron in the zinc-iron alloy layers oxidizes. The oxidizing of the free iron in the intermetallic layers results in the discoloration of the surrounding zinc coating, changing the surface appearance from the well-known grey appearance of galvanized steel to a brown color.
The formation of brown staining does not occur until the pure zinc eta-layer or most of the eta-layer matrix surrounding the zeta layer has been consumed by corrosion or removed in the local areas where the staining appears. A similar type of reaction can occur on laundry, porcelain, dishes, utensils, and even glassware when the iron is oxidized, allowing brown staining to occur.
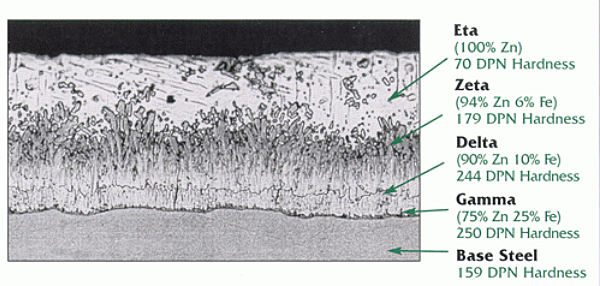
The results of laboratory tests and held surveys have shown zinc coatings applied to reactive, high-silicon steels are more susceptible to brown staining than zinc coatings applied to low silicon steels. This is primarily due to the differences observed in the structural layout of the zinc coating on the two types of steel after they have been galvanized. When low-silicon steels are galvanized, the eta-layer is relatively thick and the intermetallic structure is tightly compact, which makes it difficult for any free iron particles to penetrate the zeta-layer and become oxidized.
However, when high-silicon steels are galvanized, the eta-layer is relatively thin and the intermetallic structure is not tightly compact. The zinc-iron alloy in the zeta-layer form stall, vertical columns that are spaced in a manner which allows free iron particles to migrate to the top of the zinc coating. Once these free iron particles reach the surface of the zinc coating, they may become oxidized and form brown stains on the surface. It is sometimes difficult to visually distinguish red rust on the base steel from brown staining. However, a coating thickness gauge can be used to distinguish between the two since red rust will only form if there is no zinc present at the specific point on the product where the defect is located. If only brown stains are present, the base steel is not rusting and the corrosion performance of the galvanized steel is not affected. Since there is the presence of zinc under the brown stains, brown staining is acceptable according to the ASTM A123 specification.
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.

