Alloy Additions to the Kettle and Their Purposes
What is the purpose of each of the alloy elements that can be added to the galvanizing kettle?
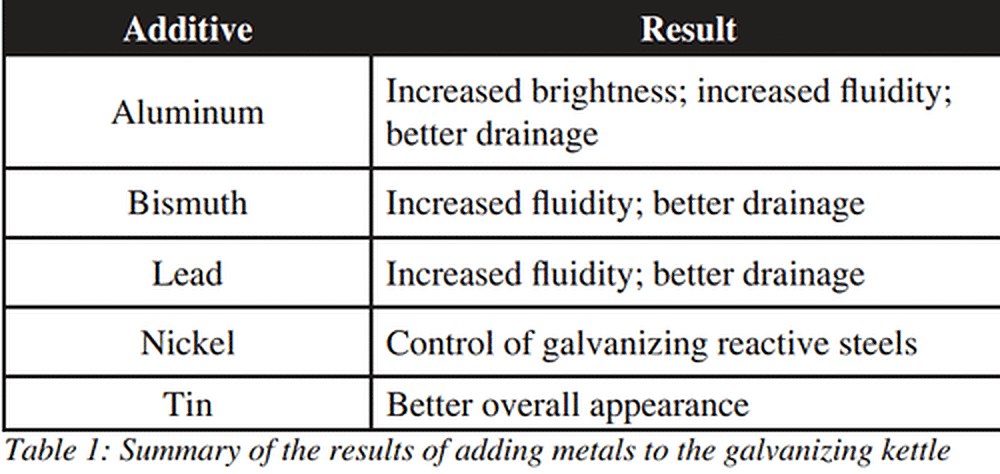
The galvanizing kettle can contain any one of three grades of zinc that are specified in ASTM B6 and are at least 98% pure. Sometimes other metals may be added to the kettle in order to promote certain desirable properties in the zinc coating. These metals include aluminum, bismuth, lead, nickel, and tin, each of which serves a different purpose in the galvanizing kettle.
Aluminum
Aluminum is less dense than zinc but has a higher melting point. When added to the galvanizing kettle, aluminum creates a thin oxide film on the surface of the bath. It is most commonly used to create a brighter coating appearance and to slightly increase the fluidity of the zinc. The increase in fluidity helps to improve the drainage of excess zinc off a product and back into the galvanizing kettle. A maximum level of 0.007% of aluminum must be maintained in the kettle in order to avoid the potential creation of bare spots on products due to reactions with the zinc ammonium chloride flux. Aluminum is difficult to maintain in the zinc bath because it floats on the zinc so it is usually added as brightener bar and plunged under the surface of the zinc to mix well.
Bismuth
Bismuth is commonly used in metal alloys with a low melting point, and is denser than zinc, but has a lower melting point. When bismuth is added to the galvanizing kettle it increases the fluidity of the zinc, which helps prevent bridged holes, clogged threads, and runs in the zinc coating. Bismuth is very stable in the zinc bath and additions are based on the amount of zinc used.
Lead
Lead is denser than zinc but has a lower melting point. When lead is added to the galvanizing kettle, it increases the fluidity of the zinc. The increase in fluidity helps to improve the drainage of excess zinc off a product and back into the galvanizing kettle. Lead is very stable in the zinc bath and is pre-alloyed in prime western zinc. This alloy element can create a lead layer at the bottom of the kettle and the drossing process may be assisted by the dross floating on the dense lead layer so the dross is easier to remove.
Nickel
Nickel is commonly used for metal alloys with a high melting point, is denser than zinc, and has a higher melting point. Nickel is most commonly added to the galvanizing kettle to help control reactive steels by reducing the intermetallic formation. This process is effective as long as the product being galvanized has a silicon level less than 0.20%. However, steels with low silicon, less than 0.03% may obtain coatings under the specified thickness. Nickel is also difficult to maintain in the zinc bath because it must be regularly added to the kettle and nickel powder additions require the use of special equipment. A critical maximum level of 0.05% of nickel must be maintained in a kettle operating at 440 C (824 F) in order to help avoid excessive dross formation.
Tin
Tin is commonly used for corrosion protection and metal alloys with a low melting point. Tin has a higher density than zinc, but a lower melting point. Tin is commonly combined with lead in a zinc bath to help modify the coating appearance. With tin, a level of at least 0.05% must be maintained in order to have an impact and to help increase spangle size and contrast in the galvanized coating. Tin is also very stable when combined with zinc and the amount added to a kettle is based on the amount of zinc used.
For more information on this issue of Dr. Galv, contact The AGA at 720-361-4485
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.

