Acid Rain Conditions
How is acid rain measured and what do we know about the corrosion protection of galvanizing in acid rain conditions?
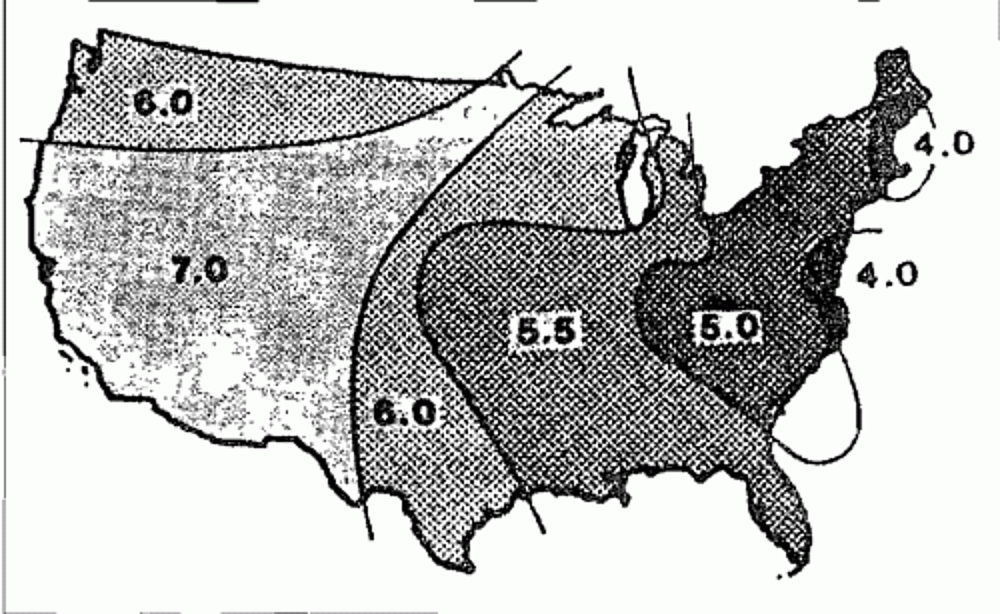
Our highly industrialized society and fast-paced lifestyle ha vet resulted in greater quantities of hydrogen atoms being distributed by wind currents or absorption and later release -by rain clouds. Chemical pollutants combine with atmospheric moisture to create acid rain. Due to heavy industrialization, major cities often have the highest levels of acid rain. Carbon monoxides from the exhausts of auto mobiles and chemicals from industry are the main contributors of the raw materials that create acid rain, but other sources include fertilizers and pesticides. The map below, compiled by the National Center for Atmospheric Studies, indicates acid rains falling in the United States vary in pH content.
The lower a pH (which means "part of hydrogen") number, the more acidic the solutions being described. A pH number identifies degree, not amount, of a solution's acidity or alkalinity.
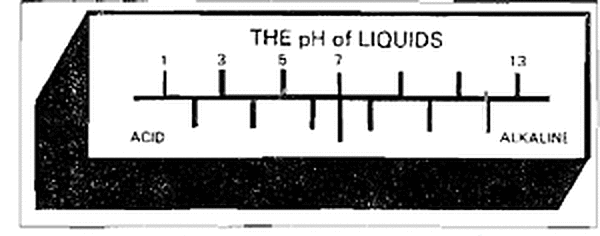
The neutral point on the pH scales is 7.0. Think of the above picture as a weighing scale which tips to the left (where numbers are lower) for acid content and to the right (higher' numbers) for alkaline content. Rain water can vary from a pH of 3.0 to a pH of 8.5, depending on meteorological conditions and the geographical location where it falls. The question of how the galvanized coating protects in acid rain conditions has no definite answers. However, if the question is asked by a prospective customer, helping them to understand the problem may be beneficial. Studies conclude that factors such as direction, velocity, surface "wetness and duration of its wetness, combined with average temperature, can be used to estimate where the effects of acid rain are likely to be more severe. These factors also provide clues to dealing with the problem.
© 2026 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.

