Different Appearances
As stated, the appearance of hot-dip galvanized steel immediately after galvanizing can be bright and shiny, spangled, matte gray, or a combination of these. There are a number of reasons for the difference in appearance, as explored here, but regardless if the piece is shiny or dull, the appearance has no effect on the corrosion performance. And in time after exposure to the environment, all galvanized coatings will take on a uniform matte gray appearance.
Reasons for Different Appearances
Steel Chemistry
The most common reason for galvanized steel to have different appearances is the chemistry of the steel pieces. There are two elements of steel chemistry which most strongly influence the final appearance; silicon and phosphorous. Both silicon and phosphorous promote coating growth, and this thicker coating is responsible for the differing appearance.
The amount of silicon added during the steel making process to deoxidize the steel can create differences in appearance of galvanized products. The recommended silicon composition for steel to be galvanized is either less than 0.04% or between 0.15% and 0.25%. Any steels not within these ranges are considered reactive steels and are expected to form thicker than average zinc coatings.
In addition to producing thicker coatings, highly reactive steels tend to have a matte gray or mottled appearance instead of the typical bright coating. This difference in appearance is a result of the rapid zinc-iron intermetallic growth that consumes all of the bright, pure zinc. This growth of the intermetallic layer is generally out of the galvanizer's control, because they usually do not have prior knowledge of the steel's composition. However, this increased coating thickness can be beneficial in some respects because time to first maintenance is directly proportional to coating thickness.
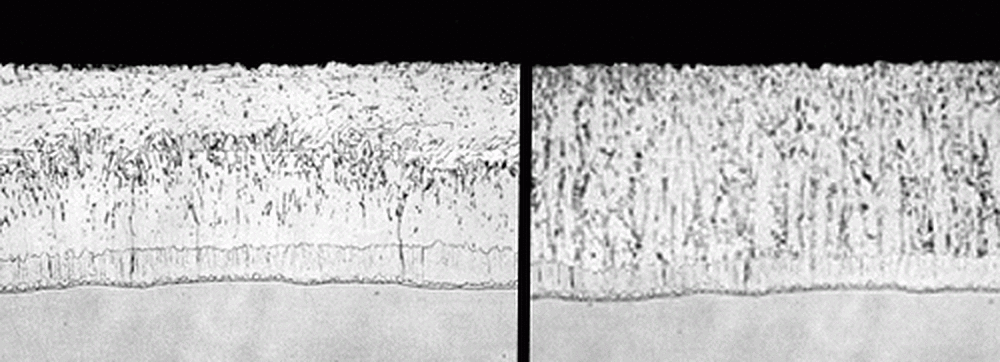
Figure 18 shows the difference in alloy formation on steel with the recommended silicon ranges (left) and those of reactive steels (right). The photomicrograph clearly shows the microscopic level differences that can occur due to the amount of silicon in the steel being hot-dip galvanized.
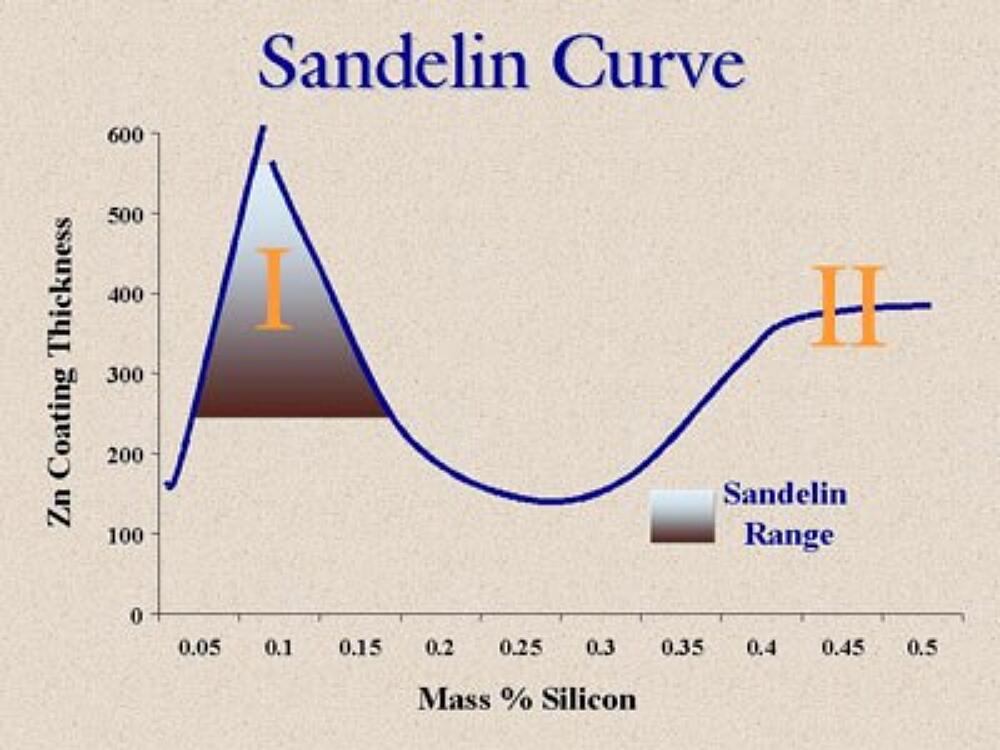
The Sandelin curve, as seen in Figure 19, compares the zinc coating thickness to the mass percentage of silicon in the steel. The range on the graph labeled "I" is called the Sandelin range, named after the metallurgist who discovered the behavior, and the coatings tend to be thick and dull gray as a direct result of the percentage of silicon present in the base steel. The Sandelin range is roughly between 0.05% and 0.15% silicon. The rangeon the graph labeled "II", which represents a steel content of greater than 0.25% silicon, shows the coating thickness increases with increased silicon content and then starts to level off at around 0.40% silicon.

Similar to silicon, the presence of phosphorus influences the reaction between the liquid zinc and the steel. Figure 20 shows steel with phosphorous levels over 0.04%, which produces matte gray coating areas and ridges of rough surfaces where there is increased intermetallic growth and a thicker coating. Phosphorus is generally considered an impurity in steel except where its beneficial effects on machinability and resistance to atmospheric corrosion are desired. Some steels such as ASTM A242 Type 1 present problems because they may contain both a high level of phosphorus and a high level of silicon.
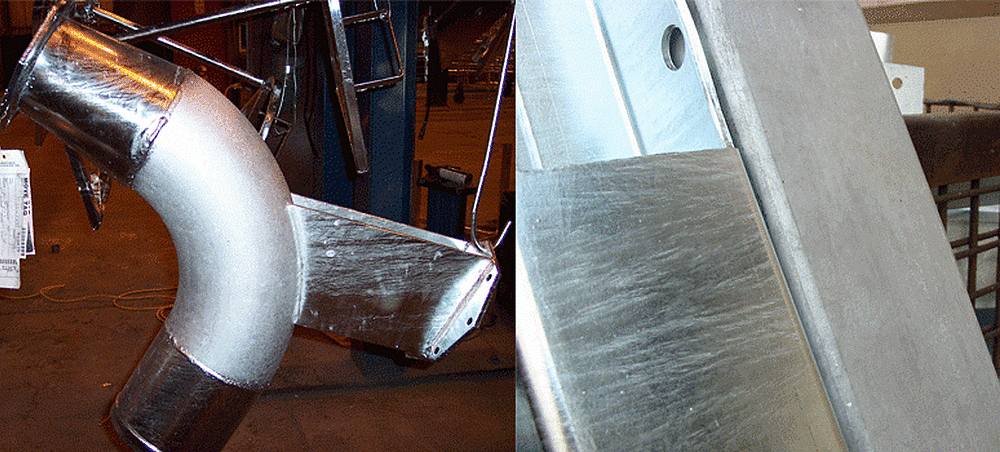
Figure 21 is an example of products with separate galvanized pieces that have very different appearances due to the difference in steel chemistry. This is often observed when connecting different types or thicknesses of steel, but can also occur on similar pieces. Another common place this is found is in welded areas, as the silicon content of the weld rod can influence appearance. Regardless of the appearance, all of these products have an equal amount of corrosion resistance and meet the specification requirements.

Cooling Rate
A steel part with both dull and shiny coating can also be the result of a different cooling rate. In Figure 22, the outer edges were cooled rapidly, which allowed free zinc or an eta layer to form on top of the intermetallic layers. The zinc in the center of the product remained above 550 F longer, thus the metallurgical reaction between zinc and iron continued consuming the free zinc layer, which gives the matte gray look. Eventually, as the product weathers, the differences in appearance will become less noticeable and/or disappear and the piece will become a uniform, matte gray throughout.

Steel Processing
In addition to temperature and chemistry of the steel, the processing of the steel can also create a bright or dull appearance in galvanized products. The top rail in Figure 23 has a winding pattern of dull gray areas corresponding to the process used during the tube making. The stresses in the steel affect the intermetallic formation and can create this striped look. The corrosion protection is not affected and these parts are acceptable.