Zinc Coatings
Do all zinc coatings provide similar corrosion protection?
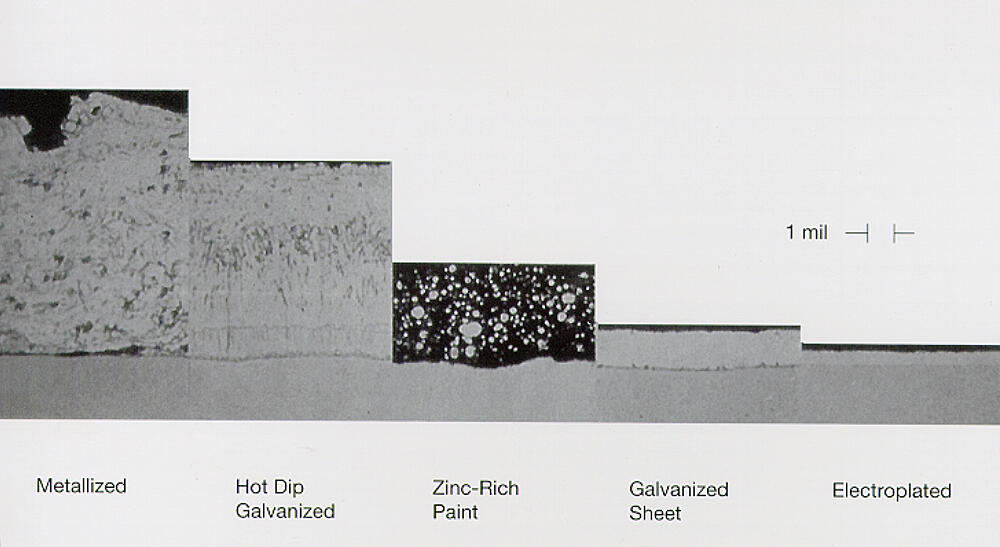
This is a common question and it can be very confusing because of all the miscommunication in the marketplace. Some suppliers call any type of zinc coating galvanized, which is incorrect and can be very misleading. Not all zinc coatings are created equal in terms of corrosion protection. Lets compare the difference between batch and continuous galvanized coatings, zinc-rich paint, mechanical plating, and metallizing.
Batch hot-dip galvanizing is the process American Galvanizers Association specializes in. This process includes degreasing the steel, pickling it to remove oxides and rust, fluxing it, and then submerging it into molten zinc. Once in the molten zinc, all corners and edges are fully covered, providing a nice uniform coating that has the same thickness on corners and edges. The steel metallurgically reacts with the steel creating three intermetallic layers and an outer coating of nearly pure zinc.
Batch hot-dip galvanizing is done on steel after-fabrication, and many types of steel are commonly galvanized using this method, including structural steel like means, sign posts, guardrail, fasteners, hardware and many other types of fabrications.
Continuous hot-dip galvanizing is another type of galvanizing, and differs from batch hot-dip galvanizing. This method is for galvanizing sheet steel, wire, and strip metal and produces a product that can be highly formed, but without the abrasion resistance of batch hot-dip galvanizing. The steel is cleaned using an alkaline solution and then is annealed. The actual galvanizing consists of running the steel through a high speed process (up to 600 feet per minute). Seven different types of products can be created using this method, each with different properties. The Galv Info Center provides services similar to the AGA, but for the continuous hot-dip galvanizing industry.
Another type of zinc coating is zinc rich paint, and is sometimes mistakenly called cold galvanizing. Zinc rich paint (ZRP) is not actually a galvanizing product. It is a paint that contains zinc dust and does not react mtallurgically with the steel, like hot-dip galvanizing does. Painting with ZRP does not provide the long service life hot-dip galvanizing does. When the paint is prepared properly, which can be difficult and depends on the expertise of the applicator, the zinc dust can provide some cathodic protection to the steel. However, the cathodic protection depends largely on the proper preparation of the paint, and the application of the product to the steel. ZRP does not offer abrasion resistance to the steel like batch hot-dip galvanizing does.
Another zinc coating mistakenly referred to as galvanizing is mechanical plating. This method is something referred to as mechanical galvanizing, and consists of coating the steel with copper and then tumbling the steel in a barrel wit glass beads and zinc powder. The beads peen the steel surface and thereby apply the zinc powder. Because of size limitations, mechanical plating is only done on fasteners and small hardware. The zinc coating obtained using this method has a density of only 0.45 oz/ft2/mil compared to the density of a hot-dip galvanizing coating. This means batch hot-dip galvanizing provides over 30% more zinc on the steel, which is directly related to also providing a longer service life. The zinc coating on the mechanically plated steel doesnt adhere as tenaciously as that hot-dip galvanizing.
One other zinc coating used to coat steel is zinc flame spray or metallizing. This method consists of using an application gun to atomize zinc powder and then propel it at the steel surface. The coating is slightly porous, and can be applied in the field or shop. Metallizing can be considerable more expensive than galvanizing and the quality of the zinc coating depends largely on the skill of the applicator.
© 2024 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.

