In Concrete

Concrete is an extremely complex material. The use of various types of concrete in construction has made the chemical, physical, and mechanical properties of concrete and its relationship to metals a topic of ongoing studies. Reinforcing steel bars (rebar) are embedded in concrete to provide strength, and are critical to the integrity and performance of the structure throughout its life. As concrete is a porous material, corrosive elements such as water, chloride ions, oxygen, carbon dioxide, and other gases travel into the concrete matrix, eventually reaching the rebar. Once the concentration of these corrosive elements surpasses steels corrosion threshold, the rebar starts to corrode. As the rebar corrodes, pressure builds around the bar leading to cracking, staining, and eventually spalling of the concrete.

Because failure of the rebar leads to compromised or failing structural capacity, protecting against premature rebar failure is key. Similar to in the atmosphere, galvanized rebar extends the life of the steel in concrete. The corrosion mechanisms in concrete are quite different than atmospheric exposure, and one of the biggest factors is chloride concentration. Galvanized rebar can withstand chloride concentration at least four to five times higher than black steel, and remains passivated at lower pH levels, slowing the rate of corrosion. In addition to the higher chloride tolerance, once zinc corrosion products are formed from the galvanized rebar, they are less voluminous than iron oxide and actually migrate away from the bar. The less voluminous zinc particles migrate away from the bar (galvanized coating) and into the pores of the concrete matrix. This migration prevents the pressure buildup and spalling caused by iron oxide particles.

The total life of galvanized steel in concrete is made up of the time taken for the zinc to depassivate, plus the time taken for consumption of the zinc coating, as it sacrificially protects the underlying steel. Only after the coating has been fully consumed in a region of the bar will localized steel corrosion begin. Laboratory data support, and field test results confirm, that reinforced concrete structures exposed to aggressive environments have a substantially longer service life when galvanized rebar is used as opposed to bare steel rebar.
Visit the Galvanized Rebar website for more information regarding market-specific information of hot-dip galvanized reinforcing steel. The Reinforcing Steel section in the AGA Publication area has many publications to learn more about using galvanized rebar. You can also download the AGA publication, Hot-Dip Galvanized Reinforcing Steel: A Specifier's Guide for more detailed information. Lastly, learn more about galvanized rebar and earn continuing education credits by requesting an in-person seminaror taking our online rebar seminar.
To learn more about concrete corrosion, follow the links below:
- Corrosion Resistance of Hot-Dip Galvanized Rebar in Concrete
- Bond Strength
- Zinc Reaction in Concrete
- Removal of Forms
- Concrete References
Galvanized Steel Stories: New York State Thruway Authority- Galvanized Reinforcing Steel
Corrosion Resistance of Galvanized Rebar in Concrete
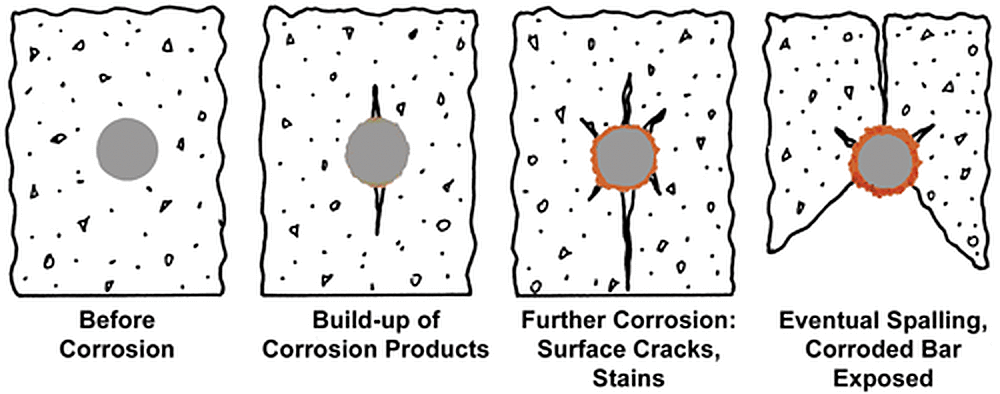
The corrosion mechanisms and performance of black and hot-dip galvanized steel in concrete are different than when exposed to atmospheric conditions. Steel embedded in concrete is exposed to a highly alkaline environment. Black steel is passive in alkaline concrete until the chloride level exceeds approximately 1 lb/yd3, when steel becomes depassivated and starts to corrode. Zinc, on the other hand, can withstand chloride concentration at least four to five times higher than black steel, and coupled with its impervious barrier protection, delays the onset of chloride corrosion on galvanized rebar.
Chlorides penetrate the concrete through small pores and cracks that form on the surface through use and weathering. While black steel in concrete typically depassivates below a pH of 11.5, galvanized reinforcement can remain passivated at a lower pH, thereby offering substantial protection against the effects of concrete carbonation.
In addition to the higher chloride tolerance, once the zinc coating does start to depassivate, the zinc corrosion products formed are less voluminous than iron oxides and actually migrate away from the galvanized bar into the matrix of the concrete. Unlike the development of iron oxide, the migration of the zinc corrosion products from the rebar prevents the pressure buildup and eventual concrete spalling.
The total life of a galvanized coating in concrete is made up of the time taken for the zinc to depassivate (which takes longer than black steel because of its higher tolerance to chloride ions), plus the time taken for the consumption of the zinc coating as it sacrificially protects the underlying steel. Only after the coating has been fully consumed in a region of the bar will localized corrosion of the steel begin.
Bond Strength
Good bonding between reinforcing steel and concrete is essential for the reliable performance of reinforced concrete structures. When protective coatings on steel are used, it is essential to ensure they do not reduce bond strength. Studies on the bonding of galvanized and black steel bars to Portland Cement concrete have been investigated. The results of these studies indicate:

- Development of the bond between black or galvanized steel and concrete depends upon cure time and environmental factors.
- In some cases, the full bond for galvanized rebar may take longer to form than for uncoated steel, depending on the zincate/cement reaction.
- As reported by Stephen Yeomans in GalvanizedSteel Reinforcement in Concrete, there are a number of studies that have concluded the fully developed bond strength of galvanized rebar has no significant difference from black rebar bond strength.
- A study by C. Andrade in Spain monitored bond strength of galvanized rebar samples over 10 years immersed in seawater and found no deleterious effects on bond strength over that time.
Bond Strength Test
The bond of the hot-dip galvanized reinforcing bar to the concrete can be tested according to ASTM A944, Test Methods for Comparing Bond Strength of Steel Reinforcing Bars to Concrete Using Beam-End Specimens. The bond strength relies heavily on the deformation of the bar and not as much on the actual bond between the zinc and the concrete. For plain bars with no deformation, the bond between the zinc and the concrete becomes very important. Pullout strength of hot-dip galvanized reinforcing steel has been tested many times, and the values of bond strength are equivalent to, or better than, black steel bond strength.
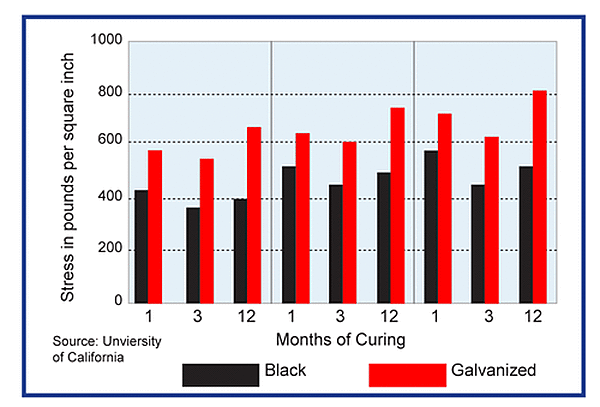
Zinc Reaction in Concrete

During curing, the galvanized surface of steel reinforcement reacts with the alkaline cement paste to form stable, insoluble zinc salts accompanied by hydrogen evolution. This has raised the concern of the possibility of steel embrittlement due to hydrogen absorption. Laboratory studies indicate liberated hydrogen does not permeate the galvanized coating to the underlying steel and the reaction ceases as soon as the concrete hardens.
ASTM A767 requires hot-dip galvanized reinforcement be chromate passivated after galvanizing. Many cement mixtures contain small amounts of chromate that may serve the same purpose as chromate passivating the zinc coating. The reaction between the alkaline cement paste and the zinc coating is dependent on the amount of zinc-coated surface in the concrete with the potential for reaction increasing with more zinc metal in contact with the concrete.
Removal of Forms

Metal forms should be electrically isolated from the galvanized rebar to prevent dissimilar metal reactions during the concrete curing. If metal forms are not isolated from the galvanized rebar, then zinc ions can be released from the galvanized coating to try to protect the metal form, resulting in a change in the concrete appearance near the galvanized bar placement.
Because types of cement with naturally low occurring levels of chromates may react with zinc, it is important to ensure that forms and supports are not removed before the concrete has developed the required strength to support itself. Normal form removal practices may be utilized if the cement contains at least 100 ppm of chromates in the final concrete mix or if the hot-dip galvanized bars are chromate-passivated according to ASTM A767.
Ductility and strength of reinforcing steel are important to prevent brittle failure of reinforced concrete. Studies of the effect of galvanizing on the mechanical properties of steel reinforcing bars have demonstrated the tensile, yield and ultimate strength, ultimate elongation, and bend requirements of steel reinforcement are substantially unaffected by hot-dip galvanizing, provided proper attention is given to steel selection, fabrication practices, and galvanizing procedures.
The effect of the galvanizing process on the ductility of steel bar anchors and inserts after being subjected to different fabrication procedures also has been investigated. The results demonstrate conclusively with the correct choice of steel and galvanizing procedures, there is no reduction in steels ductility.
Concrete References
- Additional studies and information on galvanized rebar can be found at www.galvanizedrebar.com, in the AGA publication Galvanizing for Corrosion Protection: A Specifiers Guide to Reinforcing Steel, and through these concrete references:
- Committee 222.Corrosion of Metals in Concrete; American Concrete Institute, 222R-85, 1985.
- Adnrade, C. et al. Corrosion Behavior of Galvanized Steel in Concrete; 2nd International Conference on Deterioration and Repair of Reinforced Concrete in the Arabian Gulf; Proceedings Vol. 1, pp. 395-410, 1987.
- Arup, H. The Mechanisms of the Protection of Steel by Concrete; Society of Chemical Industry Conference of Reinforcement in Concrete Construction; London, June 1983.
- Structures A Scientific Assessment; CSIRO Paper, Sydney, 1979.
- Bird, C.E. Bond of Galvanized Steel Reinforcement in Concrete; Nature, Vol. 94, No. 4380, 1962.
- Breseler B. & Cornet I. Galvanized Steel Reinforcement in Concrete; 7th Congress of the International Association of Bridge and Structural Engineers, Rio de Janeiro, 1964.
- Chandler, K.A. &Bayliss, D.A. Corrosion Protection of Steel Structures; Elsevier Applied Science Publishers, pp. 338-339, 1985.
- Cornet, I. &Breseler, B. Corrosion of Steel and Galvanized Steel in Concrete; Materials Protection, Vol. 5, No. 4, pp. 69-72, 1966.
- Concrete Institute of Australia. The Use of Galvanized Reinforcement in Concrete; Current Practice Note 17, September 1984. ISBN 0 909375 21 6.
- Duval, R. &Arliguie, G.; Memoirs Scientifiques Rev. Metallurg; LXXI, No. 11, 1974.
- Galvanizers Association of Australia.Hot Dip Galvanizing Manual; 1985.
- Hime, W. &Erlin, B. Some Chemical and Physical Aspects of Phenomena Associated with Chloride-Induced Corrosion; Corrosion, Concrete and Chlorides; Steel Corrosion in Concrete: Causes and Restraints; ACI SP-102, 1987.
- Hosfoy, A.E. &Gukild, I. Bond Studies of Hot Dipped Galvanized Reinforcement in Concrete; ACI Journal, March, pp. 174-184, 1969.
- India Lead Zinc Information Centre. Protection of Reinforcement in Concrete, An Update, Galvanizing and Other Methods; New Delhi, 1995.
- International Lead Zinc Research Organization.Galvanized Reinforcement for Concrete II; USA, 1981.
- Kinstler, J.K. Galvanized Reinforcing Steel Research, Survey and Synthesis; International Bridge Conference Special Interest Program, Pittsburgh, PA, 1995.
- MacGregor, B.R. Galvanized Solution to Rebar Corrosion; Civil Engineering, UK, 1987.
- Page, C.L. &Treadway, K.W.J. Aspects of the Electrochemistry of Steel in Concrete; Nature, V297, May 1982, pp. 109-115.
- Portland Cement Association. An Analysis of Selected Trace Metals in Cement and Kiln Dust; PCA publication SP109, 1992.
- Roberts, A.W. Bond Characteristics of Concrete Reinforcing Tendons Coated with Zinc; ILZRO Project ZE-222, 1977.
- Tonini, D.E. & Dean, S.W. Chloride Corrosion of Steel in Concrete; ASTM-STP 629, 1976.
- Warner, R.F., Rangan, B.V., & Hall, A.S. Reinforced Concrete; Longman Cheshire, 3rd edition, pp. 163-169, 1989.
- Worthington, J.C., Bonner, D.G. &Nowell, D.V. Influence of Cement Chemistry on Chloride Attack of Concrete; Material Science and Technology; pp. 305-313, 1988.
- Yeomans, S.R. & Hadley, M.B. Galvanized Reinforcement Current Practice and Developing Trend; Australian Corrosion Association Conference, Adelaide, 17 pp., November, 1986.
- Yeomans, S.R. Corrosion Behavior and Bond Strength of Galvanized Reinforcement and Epoxy Coat.
- Yeomans, S.R. "Galvanized Steel Reinforcement in Concrete." Elsevier Ltd., 2004.
