Limiting Coating Growth by Blasting
Is blasting an effective way to limit coating growth on reactive steel?
The simple answer is yes, but lets discuss why and how. When we talk about reactive steel we are talking about steel with chemistry outside the recommended limits for galvanizing. ASTM A385 recommends using steel with a silicon level less than 0.04% or between 0.15% and 0.22%, and a phosphorus level less than 0.04%. Steel with chemistry outside these conditions can develop a much thicker and more brittle coating than the normal galvanized coating. It is important to note even though a steel material certification might indicate a particular steel has the recommended chemistry for galvanizing, the certification is only a sample and the steel can have higher concentrations of elements such as silicon or phosphorus in some areas. One way to limit coating growth on reactive steel is to blast it before galvanizing, as we will discuss in this article.
Characteristics of Reactive Steel
Reactive steel poses a problem because of the potential to develop excessively thick coatings. Excessively thick coatings are those which are greater than 10 mils, and they tend to be much more brittle. This also means reactive steel can consume much more zinc and thus cost more to process. The intermetallic layers often compose all of the coating thickness on reactive steel and when the zeta layer is exposed to the environment, the galvanized coating can appear matte gray, as opposed to being bright and shiny. Coatings with thicker intermetallic layers can also have a higher probability of developing brown staining, and although this type of staining doesnt affect the time to first maintenance of the product, it can be objectionable to some end users. The coating on reactive steel tends to be a rougher surface and, in some instances, not acceptable if the steel is intended for use where it will be handled daily.
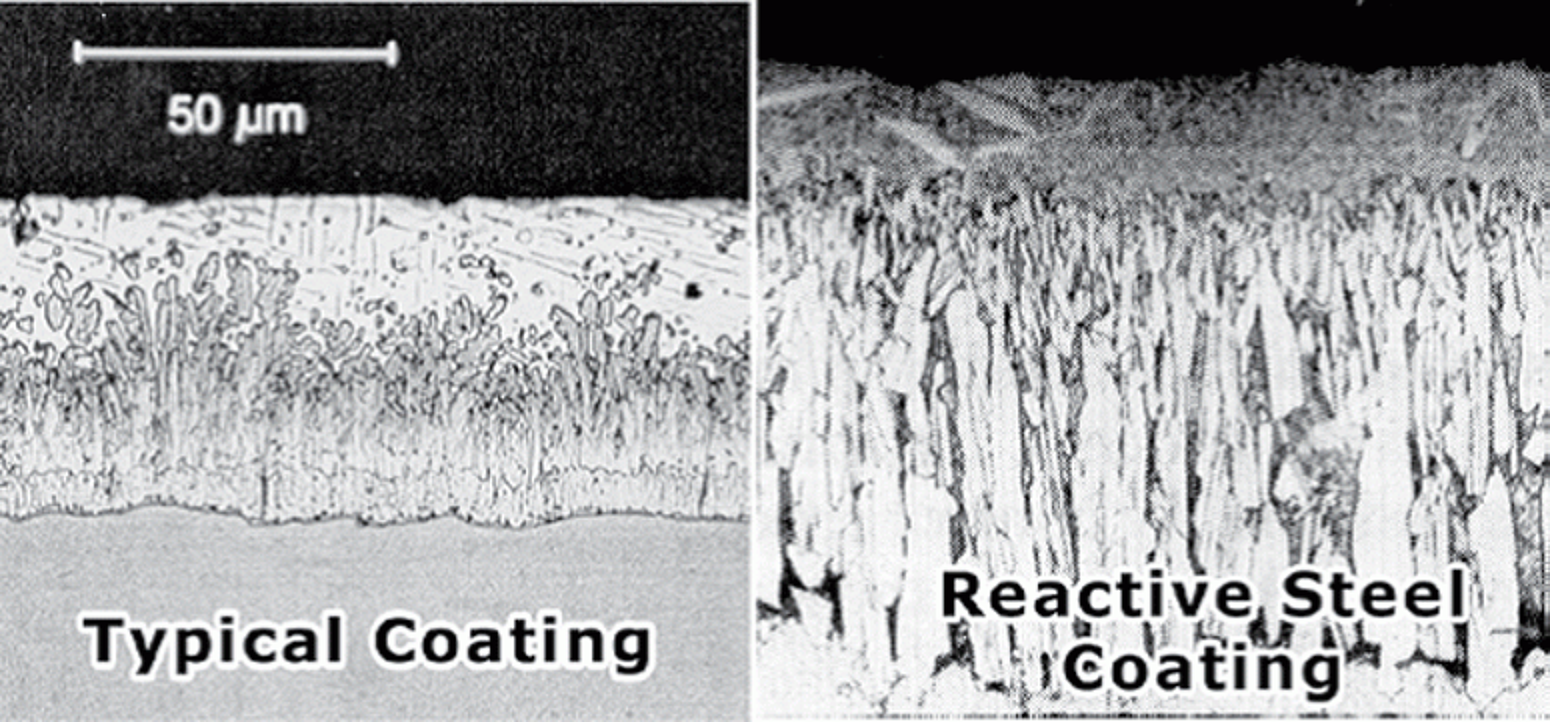
Intermetallic Layer Growth
The galvanizing reaction is a diffusion process with the layers of the coating growing perpendicularly outward from the base steel. Compared to steel with recommended chemistry for galvanizing, the intermetallic layers of reactive steel grow in long, pronounced and jagged columns as shown above. Blasting interferes with the growth of the zeta intermetallic layer of the coating.
Blasting Reactive Steel
Blasting is a process whereby abrasive particles are propelled at the steel surface using compressed air. Blasting can be done to remove paint, varnish, or sand from castings, substitute acid pickling, remove weld residues, or remove rust or mill scale from steel. Blasting can also be done on reactive steel to limit coating growth during galvanizing. Blasting roughens the surface of steel and in so doing interferes with the growth of the zeta intermetallic layer. Rather than growing in long columns when the surface is flat, blasting creates many peaks and valleys so the crystals collide and interfere with each other to the point they cannot continue growing. This interferes with how long the crystals can grow and thus limits the coating thickness. Blasting can be done with many different media, including aluminum oxide, carbon dioxide, baking soda, crushed corncobs, dry ice, glass pellets, metal shot, sand, silicon carbide, steel grit, walnut shells, and wire cuttings. However, blasting is not a replacement for sourcing steel with the recommended chemistry because blasting can add costs and delays to the galvanizing process. However, in those times when you must galvanize reactive steel, blasting can help to decrease the coating thickness.
© 2024 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use.


Comments
[Comment awaiting moderation]